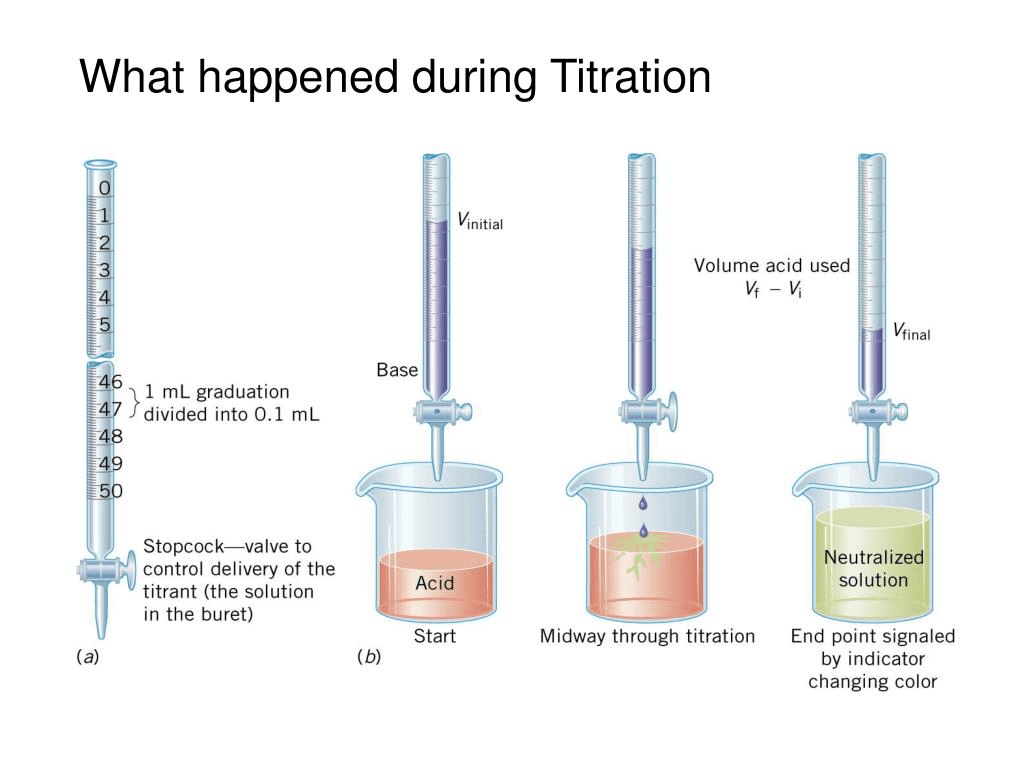
End Point Chem Def . The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The end point typically comes. The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The endpoint refers to the point in. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is.
from edurev.in
The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The end point typically comes. The endpoint refers to the point in. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached.
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev
End Point Chem Def The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint refers to the point in. The end point typically comes. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point Chem Def The end point typically comes. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Endpoint and. End Point Chem Def.
From lessonlibrarysnotter.z13.web.core.windows.net
Parts Of A Chemical Equation End Point Chem Def The endpoint refers to the point in. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an. End Point Chem Def.
From owlcation.com
Preparing for the American Chemical Society General Chemistry Exam End Point Chem Def The end point typically comes. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint refers to the point in. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Endpoint and. End Point Chem Def.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free End Point Chem Def Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The completion of a titration is the end. End Point Chem Def.
From www.conquerhsc.com
HSC Chemistry Module 6 Inquiry Question 3 End Point Chem Def Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The end point typically comes. The endpoint. End Point Chem Def.
From solvedlib.com
Fajan of halides endpoint detection with Agt ion are… SolvedLib End Point Chem Def The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. Titration involves the gradual addition of a reagent of known concentration, known as. End Point Chem Def.
From philschatz.com
AcidBase Titrations · Chemistry End Point Chem Def Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to. End Point Chem Def.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki End Point Chem Def The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as. End Point Chem Def.
From theedge.com.hk
Chemistry How To Titration The Edge End Point Chem Def The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The end point typically comes. Endpoint and stoichiometric point are two. End Point Chem Def.
From www.researchgate.net
NLGB4End point of test (8 elements) Download Scientific Diagram End Point Chem Def The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. End Point Chem Def.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Ionic equilibrium deciding on end point End Point Chem Def The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint refers to the point in. The point during a. End Point Chem Def.
From www.youtube.com
End Point Reaction in biochemistry YouTube End Point Chem Def The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. The endpoint refers to the point in. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. This information, along with the. End Point Chem Def.
From www.chemicals.co.uk
What Is a Base in Chemistry? The Chemistry Blog End Point Chem Def The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. This information, along with the known concentration and the molar relationship between the two components, is. End Point Chem Def.
From www.reddit.com
[chemistry] i want to make sure if the end point here is for hcl or ca End Point Chem Def This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. The endpoint refers to the point in. The point during a titration when an. End Point Chem Def.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev End Point Chem Def The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The end point typically comes. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. This information, along with the known concentration and the molar relationship. End Point Chem Def.
From www.animalia-life.club
Endpoint Titration End Point Chem Def The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint refers to the point in. The point during a titration when. End Point Chem Def.
From www.tessshebaylo.com
Coefficient And Subscript In A Chemical Equation Tessshebaylo End Point Chem Def The endpoint refers to the point in. The end point typically comes. The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. This information, along with the known concentration and the. End Point Chem Def.
From slideplayer.com
OECD work on manufactured nanomaterials ppt download End Point Chem Def The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The point during a titration when an indicator. End Point Chem Def.
From www.researchgate.net
End Point Analysis End Point ANCOVA Estimate With the Covariate Modeled End Point Chem Def This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint refers to the point in. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The end point typically comes. The completion of a titration is the end point,. End Point Chem Def.
From chem.libretexts.org
2.3 Functional Groups Chemistry LibreTexts End Point Chem Def The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The endpoint refers to the point in. The endpoint in a titration is the point where the reaction between the analyte. End Point Chem Def.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube End Point Chem Def The endpoint refers to the point in. The end point typically comes. This information, along with the known concentration and the molar relationship between the two components, is necessary to calculate the endpoint or. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. The main difference. End Point Chem Def.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free End Point Chem Def The end point typically comes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The endpoint of. End Point Chem Def.
From www.jagranjosh.com
CBSE Class 10 Chemistry Chapter 4 Important Questions and Answers End Point Chem Def The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. Titration involves the gradual addition of a reagent of known concentration, known. End Point Chem Def.
From in.pinterest.com
End Point Flashcard 10th Grade Science Self help book, Easy science End Point Chem Def This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. The endpoint refers to the point in. The endpoint in a titration. End Point Chem Def.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF End Point Chem Def The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint of a chemical reaction or titration refers to the point at. End Point Chem Def.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry End Point Chem Def The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. The end point typically comes. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint refers to the point in. Titration involves. End Point Chem Def.
From sciencenotes.org
Triple Point Definition Triple Point of Water End Point Chem Def The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. This information, along with the known concentration and the molar relationship between the two components,. End Point Chem Def.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Chem Def The end point typically comes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint of a chemical reaction or titration refers to the point at which a specific indicator changes color, signaling the completion of the. This information, along with the known concentration. End Point Chem Def.
From www.researchgate.net
e The end point of reaction. Download Scientific Diagram End Point Chem Def The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint. End Point Chem Def.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID End Point Chem Def The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. This information, along with the known. End Point Chem Def.
From www.clutchprep.com
The End Point Analytical Chemistry Video Clutch Prep End Point Chem Def The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint in a titration is the point where the reaction between the analyte and titrant is complete, indicating that the titration is. This information, along with the known concentration and the molar relationship between the two. End Point Chem Def.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point Chem Def The endpoint refers to the point in. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known. End Point Chem Def.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder End Point Chem Def The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Endpoint and stoichiometric point are two terms commonly used in chemical analysis and titration experiments. The endpoint in a titration is the point where the reaction between the analyte and. End Point Chem Def.
From www.storyofmathematics.com
End Point Definition & Meaning End Point Chem Def Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Endpoint and stoichiometric point are two terms commonly used. End Point Chem Def.
From chemicalengineeringworld.com
Critical Point and Triple Point Chemical Engineering World End Point Chem Def Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The completion of a titration is the end. End Point Chem Def.