Binding Energy Vs Atomic Number Graph . Use a graph of binding energy per nucleon (ben) versus mass number. A higher binding energy per nucleon indicates a higher stability. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Calculate the mass defect and binding energy for a wide range of nuclei. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Nuclear binding energy = δmc 2. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. Calculate the mass defect and binding energy for a wide range of nuclei. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. The binding energy per nucleon is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Use a graph of binding energy per nucleon (ben) versus mass number. By plotting a graph of binding energy.
from www.globalsino.com
Use a graph of binding energy per nucleon (ben) versus mass number. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Calculate the mass defect and binding energy for a wide range of nuclei. A higher binding energy per nucleon indicates a higher stability. By plotting a graph of binding energy. Calculate the mass defect and binding energy for a wide range of nuclei. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev.
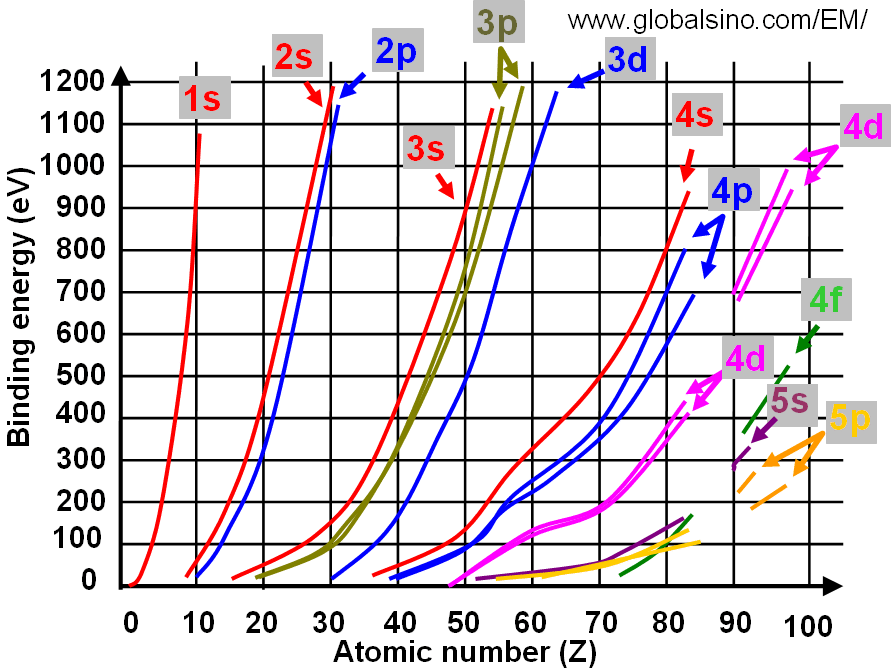
3d Elements in Periodic Table
Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Use a graph of binding energy per nucleon (ben) versus mass number. Calculate the mass defect and binding energy for a wide range of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei. By plotting a graph of binding energy. Nuclear binding energy = δmc 2. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy per nucleon is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. A higher binding energy per nucleon indicates a higher stability. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of.
From plotly.com
Atomic Number vs. Atomic Radius (pm) and First Ionization Energy (kJ Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy per nucleon is defined as: Calculate the mass defect and binding energy for a wide range of nuclei. A higher binding energy per nucleon indicates a higher stability. Iron (a = 56) has. Binding Energy Vs Atomic Number Graph.
From www.nsta.org
Focus on Physics How E = mc2 Helps Us Understand Nuclear Fission and Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Use a graph of binding energy per nucleon (ben) versus mass number. Nuclear binding energy = δmc 2. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. The binding energy (be) of a nucleus is the. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
Binding energy as a function of the nucleus mass. Download Binding Energy Vs Atomic Number Graph Calculate the mass defect and binding energy for a wide range of nuclei. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. By plotting a graph of binding energy. Calculate the mass defect and binding energy for a wide range of nuclei. Iron (a = 56) has the highest binding energy per nucleon,. Binding Energy Vs Atomic Number Graph.
From www.globalsino.com
Binding energy Practical Electron Microscopy and Database An Online Binding Energy Vs Atomic Number Graph By plotting a graph of binding energy. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Use a graph of binding energy per nucleon (ben) versus mass number. Nuclear binding energy = δmc 2. Calculate the mass defect and binding energy for a wide range of nuclei. The. Binding Energy Vs Atomic Number Graph.
From www.shutterstock.com
Nuclear Binding Energy Curve Graph Binding Stock Illustration Binding Energy Vs Atomic Number Graph Calculate the mass defect and binding energy for a wide range of nuclei. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. By plotting a graph of binding energy. Nuclear binding energy = δmc 2.. Binding Energy Vs Atomic Number Graph.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Calculate the mass defect and binding energy for a wide range of nuclei. Iron (a = 56) has the highest binding. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
4. Binding Energy per Nucleon vs. Mass Number. Download Scientific Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Calculate the mass defect and binding energy for a wide range of nuclei. By plotting a graph of binding energy. Use a graph of binding energy per nucleon (ben) versus mass number. Calculate. Binding Energy Vs Atomic Number Graph.
From www.chegg.com
Solved Generally, the more metallic an element is, the Binding Energy Vs Atomic Number Graph For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. Calculate the mass defect and binding energy for a wide range of nuclei. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Calculate the mass defect and binding energy for a wide range of nuclei. The binding energy. Binding Energy Vs Atomic Number Graph.
From chemistry.about.com
Science Clipart & Diagrams Binding Energy Vs Atomic Number Graph Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Calculate the mass defect and binding energy for a wide range of nuclei. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. A higher binding energy per nucleon indicates a higher stability.. Binding Energy Vs Atomic Number Graph.
From www.miniphysics.com
Binding Energy per Nucleon and Nuclear Stability Mini Physics Learn Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Use a graph of binding energy per nucleon (ben) versus mass number. Calculate the mass defect and binding energy for a wide range. Binding Energy Vs Atomic Number Graph.
From www.youtube.com
Binding energy graph Nuclei Physics Khan Academy YouTube Binding Energy Vs Atomic Number Graph Nuclear binding energy = δmc 2. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Use a graph of binding energy per nucleon (ben) versus mass number. For the alpha particle δm= 0.0304 u which gives a binding. Binding Energy Vs Atomic Number Graph.
From www.chegg.com
Solved 2. The graph below shows the first ionization energy Binding Energy Vs Atomic Number Graph By plotting a graph of binding energy. The binding energy of a nucleus divided by the number of nucleons in the nucleus. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Calculate the mass defect and binding energy for a wide range of nuclei. The binding energy per nucleon is. Binding Energy Vs Atomic Number Graph.
From www.chegg.com
Solved Ionization energy The graph below plots the Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. A higher binding energy per nucleon indicates a higher stability. By plotting a. Binding Energy Vs Atomic Number Graph.
From mavink.com
Electronegativity Vs Atomic Number Graph Binding Energy Vs Atomic Number Graph The binding energy per nucleon is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. By plotting a graph of binding energy. A higher binding energy per. Binding Energy Vs Atomic Number Graph.
From study.com
Binding Energy Curves & Nuclear Energy Lesson Binding Energy Vs Atomic Number Graph The binding energy per nucleon is defined as: By plotting a graph of binding energy. Use a graph of binding energy per nucleon (ben) versus mass number. Calculate the mass defect and binding energy for a wide range of nuclei. Nuclear binding energy = δmc 2. A higher binding energy per nucleon indicates a higher stability. For the alpha particle. Binding Energy Vs Atomic Number Graph.
From www.toppr.com
Draw a plot of the binding energy per nucleon as a function of mass Binding Energy Vs Atomic Number Graph For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. Nuclear binding energy = δmc 2. Calculate the mass defect and binding energy for a wide range of nuclei. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. The binding energy per nucleon is defined as: The binding. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
Binding energy per nucleon versus atomic mass number showing the Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Calculate the mass defect and binding energy for a wide range of nuclei. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. The binding energy of a nucleus divided by the number. Binding Energy Vs Atomic Number Graph.
From www.slideserve.com
PPT Trends in the periodic table PowerPoint Presentation, free Binding Energy Vs Atomic Number Graph Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Calculate the mass defect and binding energy for a wide range of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei. Nuclear binding energy = δmc 2. Use a graph of binding energy per. Binding Energy Vs Atomic Number Graph.
From courses.lumenlearning.com
Nuclear Structure and Stability Chemistry Binding Energy Vs Atomic Number Graph The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Nuclear binding energy = δmc 2. Use a graph of binding energy per nucleon (ben) versus mass number. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. By plotting. Binding Energy Vs Atomic Number Graph.
From www.sprawls.org
Characteristics and Structure of Matter Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. By plotting a graph. Binding Energy Vs Atomic Number Graph.
From ch302.cm.utexas.edu
bindingenergy Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. Calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Iron (a = 56). Binding Energy Vs Atomic Number Graph.
From marketbusinessnews.com
The difference between nuclear fission and nuclear fusion Binding Energy Vs Atomic Number Graph Nuclear binding energy = δmc 2. The binding energy per nucleon is defined as: Calculate the mass defect and binding energy for a wide range of nuclei. A higher binding energy per nucleon indicates a higher stability. By plotting a graph of binding energy. Use a graph of binding energy per nucleon (ben) versus mass number. Let's explore the graph. Binding Energy Vs Atomic Number Graph.
From www.tessshebaylo.com
Binding Energy Equation Physics Tessshebaylo Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. The binding energy per nucleon is defined as: Calculate the mass defect and binding energy for a wide range of nuclei. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. By plotting a graph of binding energy. Nuclear. Binding Energy Vs Atomic Number Graph.
From philschatz.com
Binding Energy · Physics Binding Energy Vs Atomic Number Graph Calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy of a nucleus divided by the number of nucleons in the nucleus. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Nuclear. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
Binding energy per nucleon versus atomic mass number A. Download Binding Energy Vs Atomic Number Graph For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Calculate the mass defect and binding energy for a. Binding Energy Vs Atomic Number Graph.
From courses.lumenlearning.com
Periodic Variations in Element Properties General Chemistry Binding Energy Vs Atomic Number Graph By plotting a graph of binding energy. Nuclear binding energy = δmc 2. Calculate the mass defect and binding energy for a wide range of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. A higher. Binding Energy Vs Atomic Number Graph.
From general.chemistrysteps.com
Nuclear Fission and Fusion Chemistry Steps Binding Energy Vs Atomic Number Graph Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Calculate the mass defect and binding energy for a wide range of nuclei. The binding energy per nucleon is defined as: Calculate the mass defect and binding energy for. Binding Energy Vs Atomic Number Graph.
From www.toppr.com
Draw a plot of the binding energy per nucleon as a function of mass Binding Energy Vs Atomic Number Graph The binding energy per nucleon is defined as: Nuclear binding energy = δmc 2. The binding energy of a nucleus divided by the number of nucleons in the nucleus. Use a graph of binding energy per nucleon (ben) versus mass number. A higher binding energy per nucleon indicates a higher stability. By plotting a graph of binding energy. Calculate the. Binding Energy Vs Atomic Number Graph.
From tardigrade.in
The curve of binding energy per nucleon as a function of an atomic mass Binding Energy Vs Atomic Number Graph By plotting a graph of binding energy. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Calculate the mass defect and binding energy for a wide range of nuclei. Nuclear binding energy = δmc 2. Use a graph of binding energy per nucleon (ben) versus mass number. Let's. Binding Energy Vs Atomic Number Graph.
From www.globalsino.com
3d Elements in Periodic Table Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Let's explore the graph of binding energy per nucleon vs mass number, and make predictions of. The binding energy per nucleon is defined as: Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. By plotting a. Binding Energy Vs Atomic Number Graph.
From www.slideserve.com
PPT Chapter 4 The Periodic Table PowerPoint Presentation ID6474399 Binding Energy Vs Atomic Number Graph A higher binding energy per nucleon indicates a higher stability. Nuclear binding energy = δmc 2. By plotting a graph of binding energy. Calculate the mass defect and binding energy for a wide range of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei. For the alpha particle δm= 0.0304 u which gives a binding. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
Nuclear binding energy per nucleon Download Table Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. Use a graph of binding energy per nucleon (ben) versus mass number. The binding energy (be) of a nucleus is the energy needed to separate it into. Binding Energy Vs Atomic Number Graph.
From web.mit.edu
22.02 Home [web.mit.edu] Binding Energy Vs Atomic Number Graph Use a graph of binding energy per nucleon (ben) versus mass number. Iron (a = 56) has the highest binding energy per nucleon, which makes it the most stable of all the elements. The binding energy of a nucleus divided by the number of nucleons in the nucleus. The binding energy per nucleon is defined as: Calculate the mass defect. Binding Energy Vs Atomic Number Graph.
From www.researchgate.net
The XPS analysis to estimation of binding energy of atoms and Binding Energy Vs Atomic Number Graph Calculate the mass defect and binding energy for a wide range of nuclei. For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Use a graph of binding energy per nucleon (ben) versus mass number. Use. Binding Energy Vs Atomic Number Graph.
From chem.libretexts.org
21.5 Energy Changes in Nuclear Reactions Chemistry LibreTexts Binding Energy Vs Atomic Number Graph For the alpha particle δm= 0.0304 u which gives a binding energy of 28.3 mev. The binding energy per nucleon is defined as: Calculate the mass defect and binding energy for a wide range of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei. Iron (a = 56) has the highest binding energy per nucleon,. Binding Energy Vs Atomic Number Graph.