Define Titration Of Weak Acid . learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems.
from studylib.net
titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid.
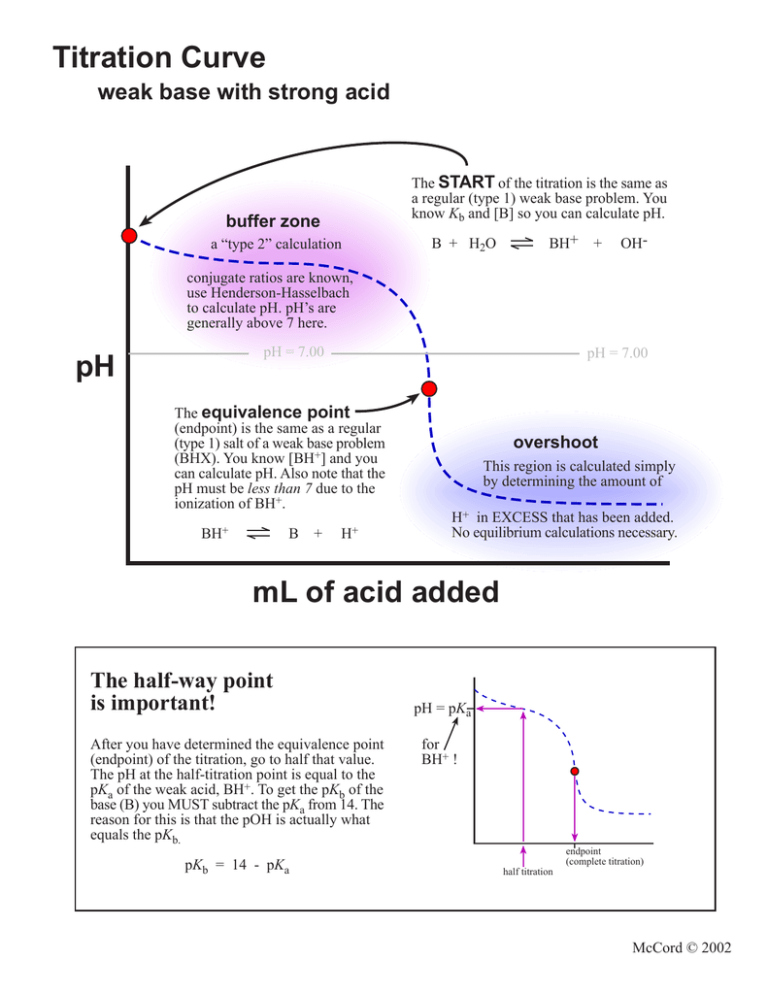
Titration Curve weak base with strong acid START
Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems.
From slidetodoc.com
1 Titration of Weak acids Titration of a Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to. Define Titration Of Weak Acid.
From www.numerade.com
SOLVED The four diagrams below represent solutions at various stages Define Titration Of Weak Acid learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. titration calculations for weak acids and strong bases involve different approaches, depending on the primary. Define Titration Of Weak Acid.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Define Titration Of Weak Acid the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems.. Define Titration Of Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn. Define Titration Of Weak Acid.
From slideplayer.com
Weak Acid Strong Base Titration ppt download Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. titration curves. Define Titration Of Weak Acid.
From www.slideshare.net
pH Understanding titration curve Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. titration curves. Define Titration Of Weak Acid.
From slidetodoc.com
Titrations of Weak Acids and Weak Bases Chapter Define Titration Of Weak Acid the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. titration curves for weak acid v weak base the common example of this. Define Titration Of Weak Acid.
From www.youtube.com
Weak Acid with Strong Base Titration 1 YouTube Define Titration Of Weak Acid learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the first example involves a strong acid titration that requires only stoichiometric calculations to derive. Define Titration Of Weak Acid.
From www.studocu.com
CH 263 S14 Titration of Weak Acids Part 2 Burand V1 Titration of Weak Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong. Define Titration Of Weak Acid.
From chem.libretexts.org
14.7 AcidBase Titrations Chemistry LibreTexts Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a. Define Titration Of Weak Acid.
From slidetodoc.com
1 Titration of Weak acids Titration of a Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. the first example involves a strong acid titration that requires only stoichiometric calculations to derive. Define Titration Of Weak Acid.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID6787925 Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn. Define Titration Of Weak Acid.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid.. Define Titration Of Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points. Define Titration Of Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral. Define Titration Of Weak Acid.
From slideplayer.com
Titration of Weak acids ppt download Define Titration Of Weak Acid learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration curves for weak acid v weak base the common example of this would. Define Titration Of Weak Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a. Define Titration Of Weak Acid.
From slidetodoc.com
1 Titration of Weak acids Titration of a Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points of. Define Titration Of Weak Acid.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points. Define Titration Of Weak Acid.
From thechemistrynotes.com
Acidbase Titration 4 Types, Theory, Working Principle Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. the titration of a weak acid like acetic acid with a weak base like aqueous. Define Titration Of Weak Acid.
From www.youtube.com
Acid/Base Theory Part V Titration of Weak Acid with Strong Base Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant.. Define Titration Of Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Define Titration Of Weak Acid the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to calculate the molarity and acidity of a weak base solution at various points of. Define Titration Of Weak Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points of. Define Titration Of Weak Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Define Titration Of Weak Acid learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. titration curves for weak acid v weak base the common example of this. Define Titration Of Weak Acid.
From www.chm.uri.edu
CHM 112 Lecture 19 Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral. Define Titration Of Weak Acid.
From studylib.net
Titration Curve weak base with strong acid START Define Titration Of Weak Acid the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to calculate the molarity and acidity of a weak base solution at various points of a. Define Titration Of Weak Acid.
From www.youtube.com
Titration weak acid calculations 2017 YouTube Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn. Define Titration Of Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn. Define Titration Of Weak Acid.
From slidetodoc.com
1 Titration of Weak acids Titration of a Define Titration Of Weak Acid titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. learn how to calculate the molarity and acidity of a weak base solution at various points of. Define Titration Of Weak Acid.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. titration curves for weak acid v weak base the common example of this would be ethanoic acid and ammonia. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the. Define Titration Of Weak Acid.
From slidetodoc.com
1 Titration of Weak acids Titration of a Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to calculate the molarity and acidity of a weak base solution at various points of a titration with a strong acid.. Define Titration Of Weak Acid.
From www.slideserve.com
PPT Titrations of Weak Acids and Weak Bases PowerPoint Presentation Define Titration Of Weak Acid learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate.. Define Titration Of Weak Acid.
From webmis.highland.cc.il.us
AcidBase Titrations Define Titration Of Weak Acid the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. learn how to perform and analyze the titration of a weak acid with a strong base, with examples and problems. the titration. Define Titration Of Weak Acid.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Define Titration Of Weak Acid titration calculations for weak acids and strong bases involve different approaches, depending on the primary reactant. the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn. Define Titration Of Weak Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Define Titration Of Weak Acid the titration of a weak acid like acetic acid with a weak base like aqueous ammonia produces a neutral ammonium acetate. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. learn how to perform and analyze the titration of a weak acid with a strong base, with examples. Define Titration Of Weak Acid.