Can The Same Drug Have Different Ndc . If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. The ndc code can be found on the outside packaging of the drug. Each finished drug product or unfinished drug subject to the listing requirements of this. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. The ndc for a drug is a numeric code. You can search with this number to find the exact drug you have. Verification of ndc assignment is not typically part of the review. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Ndc assignment is a separate process from the drug approval process.
from www.slideserve.com
Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Ndc assignment is a separate process from the drug approval process. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. Verification of ndc assignment is not typically part of the review. Each finished drug product or unfinished drug subject to the listing requirements of this. The ndc for a drug is a numeric code. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. You can search with this number to find the exact drug you have. The ndc code can be found on the outside packaging of the drug.
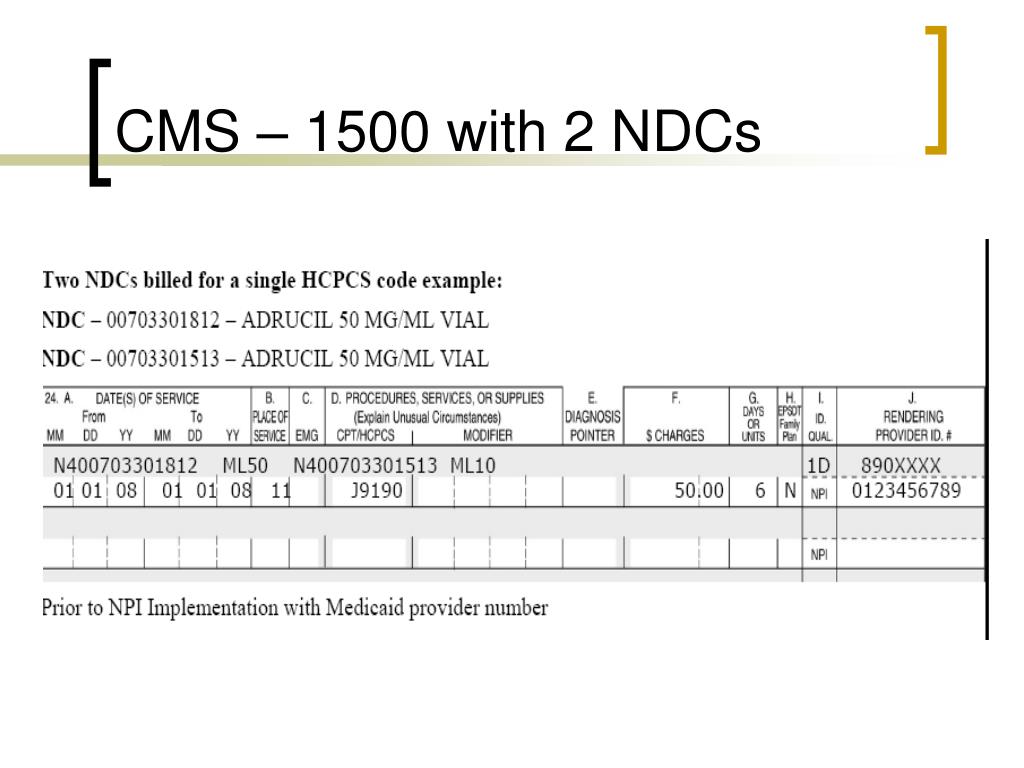
PPT NC Medicaid National Drug Code (NDC) Seminar PowerPoint Presentation ID267964
Can The Same Drug Have Different Ndc Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Each finished drug product or unfinished drug subject to the listing requirements of this. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. You can search with this number to find the exact drug you have. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Verification of ndc assignment is not typically part of the review. The ndc code can be found on the outside packaging of the drug. The ndc for a drug is a numeric code. Ndc assignment is a separate process from the drug approval process. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a.
From courses.lumenlearning.com
Mechanisms of Antibacterial Drugs Microbiology Can The Same Drug Have Different Ndc Ndc assignment is a separate process from the drug approval process. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Each finished drug product or unfinished drug subject to the listing requirements of this. The ndc for a drug is a numeric code. The ndc code can be found on the. Can The Same Drug Have Different Ndc.
From www.fda.gov
Proposed Rule on Revising the National Drug Code Format FDA Can The Same Drug Have Different Ndc Each finished drug product or unfinished drug subject to the listing requirements of this. Ndc assignment is a separate process from the drug approval process. The ndc code can be found on the outside packaging of the drug. Verification of ndc assignment is not typically part of the review. The ndc for a drug is a numeric code. Drug establishments. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT NC Medicaid National Drug Code (NDC) Seminar PowerPoint Presentation ID267964 Can The Same Drug Have Different Ndc Verification of ndc assignment is not typically part of the review. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. Ndc assignment is a separate process from the drug approval process. You can search with this number to find the exact drug you have. The ndc code can be found on. Can The Same Drug Have Different Ndc.
From slideplayer.com
Big Data Analytics in USA and Turkey ppt download Can The Same Drug Have Different Ndc Ndc assignment is a separate process from the drug approval process. Each finished drug product or unfinished drug subject to the listing requirements of this. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all. Can The Same Drug Have Different Ndc.
From i-base.info
Antiretroviral drugs illustrated pill chart Guides HIV iBase Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. Verification of ndc assignment is not typically part of the review. Each finished drug product or unfinished drug subject to the listing requirements of this. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. The ndc code can. Can The Same Drug Have Different Ndc.
From www.vrogue.co
Classification Of Drugs Drug Types And Drugs Chemical vrogue.co Can The Same Drug Have Different Ndc Ndc assignment is a separate process from the drug approval process. The ndc code can be found on the outside packaging of the drug. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. You can search with this number to find the exact drug you. Can The Same Drug Have Different Ndc.
From www.regulatoryaffairsnews.com
USFDA Revising the National Drug Code Format and Drug Label Barcode Requirements Can The Same Drug Have Different Ndc Each finished drug product or unfinished drug subject to the listing requirements of this. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Generically, equivalent drugs (the. Can The Same Drug Have Different Ndc.
From news.uga.edu
Patientfriendly prescription labels help patients take medications Can The Same Drug Have Different Ndc Verification of ndc assignment is not typically part of the review. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. My understanding is that a brand drug and a. Can The Same Drug Have Different Ndc.
From www.northwestfamilyclinics.com
News & Events Can The Same Drug Have Different Ndc Each finished drug product or unfinished drug subject to the listing requirements of this. The ndc for a drug is a numeric code. The ndc code can be found on the outside packaging of the drug. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Generically, equivalent drugs (the same drug,. Can The Same Drug Have Different Ndc.
From www.researchgate.net
Frequency of the medication National Drug Code incorrectly predicted by... Download Scientific Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Ndc assignment is a separate process from the drug approval process. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current. Can The Same Drug Have Different Ndc.
From www.visualistan.com
25 Common Medications The Same Drug, Different Names? infographic Visualistan Can The Same Drug Have Different Ndc If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Each finished drug product or unfinished drug subject to the listing requirements of this. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have.. Can The Same Drug Have Different Ndc.
From www.farwestreturns.com
National Drug Code (NDC) Far West Returns Can The Same Drug Have Different Ndc If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current. Can The Same Drug Have Different Ndc.
From www.reddit.com
Same drug, same NDC, different graphics, different manufacturers r/pharmacy Can The Same Drug Have Different Ndc Verification of ndc assignment is not typically part of the review. The ndc for a drug is a numeric code. Each finished drug product or unfinished drug subject to the listing requirements of this. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Ndc assignment is a. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT National Drug Code (NDC) PowerPoint Presentation, free download ID4414874 Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. Each finished drug product or unfinished drug subject to the listing requirements of this. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Drug establishments producing active pharmaceutical ingredients are required to provide fda. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1950891 Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. My understanding is that a brand drug. Can The Same Drug Have Different Ndc.
From www.msdmanuals.com
Overview of Response to Drugs Drugs MSD Manual Consumer Version Can The Same Drug Have Different Ndc Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. The ndc code can be found on the outside packaging of the drug. Ndc assignment is a separate. Can The Same Drug Have Different Ndc.
From www.americanhealthpackaging.com
American Health Packaging NDC Updates Can The Same Drug Have Different Ndc The ndc for a drug is a numeric code. Verification of ndc assignment is not typically part of the review. Each finished drug product or unfinished drug subject to the listing requirements of this. You can search with this number to find the exact drug you have. Ndc assignment is a separate process from the drug approval process. My understanding. Can The Same Drug Have Different Ndc.
From www.youtube.com
Pharmaceutical Dosage Forms Dosage Forms of Drugs Different Types of Dosage Forms Can The Same Drug Have Different Ndc If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. The ndc for a drug is a numeric code. Each finished drug product or. Can The Same Drug Have Different Ndc.
From www.ezmedlearning.com
Antihypertensive Medication Chart Drug Classes, List of Examples, Mechanism of Action Can The Same Drug Have Different Ndc Verification of ndc assignment is not typically part of the review. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. The ndc code can be found on. Can The Same Drug Have Different Ndc.
From www.linkedin.com
National Drug Code (NDC) Can The Same Drug Have Different Ndc My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Each finished drug product or unfinished drug subject to the listing requirements of this. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. The ndc code can. Can The Same Drug Have Different Ndc.
From rfxcel.com
FDA National Drug Code Proposed Format Changes & Industry Impact Can The Same Drug Have Different Ndc If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Verification of ndc assignment is not typically part of the review. You can search with this number to find the exact drug you have. The ndc for a drug is a numeric code. Generically, equivalent drugs (the same. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT NC Medicaid National Drug Code (NDC) Seminar PowerPoint Presentation ID267964 Can The Same Drug Have Different Ndc The ndc for a drug is a numeric code. The ndc code can be found on the outside packaging of the drug. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Verification of ndc assignment is not typically part of the review. Drug establishments producing active pharmaceutical. Can The Same Drug Have Different Ndc.
From medicalxpress.com
Why prescription drugs can work differently for different people Can The Same Drug Have Different Ndc Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. Each finished drug product or unfinished drug subject to the listing requirements of this. Verification of ndc assignment is not typically part of the. Can The Same Drug Have Different Ndc.
From www.detox.net
What are the different types of prescription drugs? Can The Same Drug Have Different Ndc The ndc for a drug is a numeric code. Verification of ndc assignment is not typically part of the review. The ndc code can be found on the outside packaging of the drug. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Ndc assignment is. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT New National Drug Code (NDC) Billing Requirement for Pharmacy Claims Submissions (UB04 Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Each finished drug product or. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT National Drug Code (NDC) PowerPoint Presentation, free download ID4414874 Can The Same Drug Have Different Ndc Verification of ndc assignment is not typically part of the review. Each finished drug product or unfinished drug subject to the listing requirements of this. Ndc assignment is a separate process from the drug approval process. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. My understanding is that a brand. Can The Same Drug Have Different Ndc.
From pharmacyfreak.com
CLASSIFICATION OF NON STEROIDAL ANTI INFLAMMATORY DRUGS Pharmacy Freak Can The Same Drug Have Different Ndc My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Generically, equivalent drugs (the same drug, concentration, and dosage form) from two different manufacturers have two different ndc. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs.. Can The Same Drug Have Different Ndc.
From ndclist.com
NDC to RxNorm Crosswalk 50580753 Zyrtec Tablet, Chewable Oral Can The Same Drug Have Different Ndc The ndc code can be found on the outside packaging of the drug. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. The ndc for a drug is a. Can The Same Drug Have Different Ndc.
From www.docsity.com
Drug Name and National Drug Code (NDC) Reference Data Summaries Law Docsity Can The Same Drug Have Different Ndc Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Verification of ndc assignment is not typically part of the review. Generically, equivalent drugs (the same drug, concentration, and dosage. Can The Same Drug Have Different Ndc.
From www.slideserve.com
PPT National Drug Code (NDC) PowerPoint Presentation, free download ID4414874 Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Drug establishments producing active pharmaceutical ingredients are required to provide fda with a current list of all drugs. The ndc code can be. Can The Same Drug Have Different Ndc.
From slideplayer.com
Depending on which drug coding system is used, cefazolin could be represented in a variety of Can The Same Drug Have Different Ndc Ndc assignment is a separate process from the drug approval process. The ndc code can be found on the outside packaging of the drug. You can search with this number to find the exact drug you have. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Drug. Can The Same Drug Have Different Ndc.
From fadic.net
Anticoagulant Drugs Commonly Used and Newer Agents Can The Same Drug Have Different Ndc The ndc for a drug is a numeric code. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Each finished drug product or unfinished drug subject to the listing requirements of this. Verification of ndc assignment is not typically part of the review. Ndc assignment. Can The Same Drug Have Different Ndc.
From www.go.netline.com
National Drug Code (NDC) Assignment Guide Free eGuide Can The Same Drug Have Different Ndc You can search with this number to find the exact drug you have. Each finished drug product or unfinished drug subject to the listing requirements of this. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Verification of ndc assignment is not typically part of. Can The Same Drug Have Different Ndc.
From www.ezmedlearning.com
Antibiotic Class Chart and Drug Name List Pharmacology Mnemonic — EZmed Can The Same Drug Have Different Ndc The ndc code can be found on the outside packaging of the drug. My understanding is that a brand drug and a generic drug with the same (active ingredient + strength + dose form) will always have. Each finished drug product or unfinished drug subject to the listing requirements of this. You can search with this number to find the. Can The Same Drug Have Different Ndc.
From www.fdabasics.com
NDC Number format pay for NDC number Can The Same Drug Have Different Ndc The ndc code can be found on the outside packaging of the drug. The ndc for a drug is a numeric code. Ndc assignment is a separate process from the drug approval process. If a single manufacturer issues the same medication in packages of different sizes (25 tablets, 50 tablets, etc.), each size has a. Verification of ndc assignment is. Can The Same Drug Have Different Ndc.