Medical Device Regulations Tga . Regulatory changes for software based medical devices. This blog compares the criteria for medical device security standards outlined by the u.s. Appropriate conformity assessment procedures in place for the device,. Reclassification of active medical devices for therapy with a diagnostic function. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Food and drug administration (fda), the european. 11.45 programmed or programmable medical device or software that is a medical device—classification rules 25 feb 2021(new inclusions) changes: Personalised medical devices (pmd) commencing: Reduced scope of custom made. The australian register of therapeutic goods (artg) is the central database of therapeutic. Therapeutic goods (medical devices) regulations 2002. Medical device manufacturers (including ivds) in australia need: Department of health and aged care.
from www.tga.gov.au
The australian register of therapeutic goods (artg) is the central database of therapeutic. 25 feb 2021(new inclusions) changes: Therapeutic goods (medical devices) regulations 2002. Medical device manufacturers (including ivds) in australia need: This blog compares the criteria for medical device security standards outlined by the u.s. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Personalised medical devices (pmd) commencing: Food and drug administration (fda), the european. Reclassification of active medical devices for therapy with a diagnostic function. Appropriate conformity assessment procedures in place for the device,.
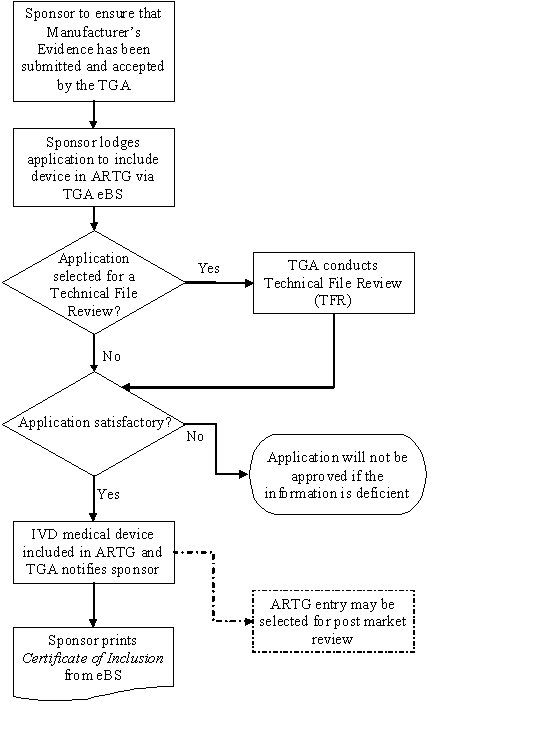
Including IVD medical devices in the ARTG Therapeutic Goods Administration (TGA)
Medical Device Regulations Tga Reduced scope of custom made. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Appropriate conformity assessment procedures in place for the device,. Therapeutic goods (medical devices) regulations 2002. Personalised medical devices (pmd) commencing: Food and drug administration (fda), the european. Reclassification of active medical devices for therapy with a diagnostic function. Department of health and aged care. 25 feb 2021(new inclusions) changes: Reduced scope of custom made. This blog compares the criteria for medical device security standards outlined by the u.s. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Regulatory changes for software based medical devices. The australian register of therapeutic goods (artg) is the central database of therapeutic. Medical device manufacturers (including ivds) in australia need:
From operonstrategist.com
TGA publishes latest medical device application processing times Medical Device Regulations Tga Regulatory changes for software based medical devices. Medical device manufacturers (including ivds) in australia need: Reclassification of active medical devices for therapy with a diagnostic function. The australian register of therapeutic goods (artg) is the central database of therapeutic. Food and drug administration (fda), the european. 11.45 programmed or programmable medical device or software that is a medical device—classification rules. Medical Device Regulations Tga.
From www.vrogue.co
Australia Tga Approval Process For Medical Devices vrogue.co Medical Device Regulations Tga Reduced scope of custom made. 25 feb 2021(new inclusions) changes: This blog compares the criteria for medical device security standards outlined by the u.s. Personalised medical devices (pmd) commencing: Regulatory changes for software based medical devices. Medical device manufacturers (including ivds) in australia need: Department of health and aged care. The australian register of therapeutic goods (artg) is the central. Medical Device Regulations Tga.
From www.regdesk.co
TGA Medical Devices Essential Principles Checklist RegDesk Medical Device Regulations Tga Department of health and aged care. This blog compares the criteria for medical device security standards outlined by the u.s. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Reclassification of active medical devices for therapy with a diagnostic function. Regulatory. Medical Device Regulations Tga.
From www.presentationeze.com
Medical Device Regulation AustraliaPresentationEZE Medical Device Regulations Tga 11.45 programmed or programmable medical device or software that is a medical device—classification rules To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. This blog compares the criteria for medical device security standards outlined by the u.s. Reduced scope of custom. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidance on Classification of Active Medical Devices Basics RegDesk Medical Device Regulations Tga This blog compares the criteria for medical device security standards outlined by the u.s. Appropriate conformity assessment procedures in place for the device,. Therapeutic goods (medical devices) regulations 2002. Reduced scope of custom made. Personalised medical devices (pmd) commencing: The australian register of therapeutic goods (artg) is the central database of therapeutic. Food and drug administration (fda), the european. Medical. Medical Device Regulations Tga.
From www.vrogue.co
Australia Tga Approval Process For Medical Devices vrogue.co Medical Device Regulations Tga To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. This blog compares the criteria for medical device security standards outlined by the u.s. Reclassification of active medical devices for therapy with a diagnostic function. The australian register of therapeutic goods (artg). Medical Device Regulations Tga.
From www.vickipartridge.com
TGA Approval For Medical Devices Australia Medical Device Regulations Tga Appropriate conformity assessment procedures in place for the device,. Therapeutic goods (medical devices) regulations 2002. The australian register of therapeutic goods (artg) is the central database of therapeutic. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. 11.45 programmed or programmable. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidelines of Regulation of SoftwareBased Medical Devices RegDesk Medical Device Regulations Tga 11.45 programmed or programmable medical device or software that is a medical device—classification rules To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Appropriate conformity assessment procedures in place for the device,. This blog compares the criteria for medical device security. Medical Device Regulations Tga.
From www.emergobyul.com
Australia TGA Approval Process for Medical Devices Medical Device Regulations Tga 11.45 programmed or programmable medical device or software that is a medical device—classification rules 25 feb 2021(new inclusions) changes: To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. The australian register of therapeutic goods (artg) is the central database of therapeutic.. Medical Device Regulations Tga.
From www.onlinegmptraining.com
Medical Device Regulations FDA, TGA, EMA etc Medical Device Regulations Tga Regulatory changes for software based medical devices. This blog compares the criteria for medical device security standards outlined by the u.s. Reduced scope of custom made. Department of health and aged care. Food and drug administration (fda), the european. Reclassification of active medical devices for therapy with a diagnostic function. 25 feb 2021(new inclusions) changes: Appropriate conformity assessment procedures in. Medical Device Regulations Tga.
From www.regdesk.co
TGA Declaration of Conformity for Class I Medical Devices RegDesk Medical Device Regulations Tga Food and drug administration (fda), the european. 25 feb 2021(new inclusions) changes: Department of health and aged care. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. This blog compares the criteria for medical device security standards outlined by the u.s.. Medical Device Regulations Tga.
From www.regdesk.co
Australian Regulatory Guidelines for Medical Devices RegDesk Medical Device Regulations Tga This blog compares the criteria for medical device security standards outlined by the u.s. Reclassification of active medical devices for therapy with a diagnostic function. Therapeutic goods (medical devices) regulations 2002. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Regulatory changes for software based medical devices. The australian register of therapeutic goods (artg) is. Medical Device Regulations Tga.
From www.regdesk.co
TGA on Regulatory Changes for SoftwareBased Medical Devices RegDesk Medical Device Regulations Tga Medical device manufacturers (including ivds) in australia need: Appropriate conformity assessment procedures in place for the device,. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Department of health and aged care. Reclassification of active medical devices for therapy with a. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidance on New Classification Rules for SoftwareBased Medical Devices (Diagnosing and Medical Device Regulations Tga Regulatory changes for software based medical devices. The australian register of therapeutic goods (artg) is the central database of therapeutic. Reclassification of active medical devices for therapy with a diagnostic function. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Department. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidance on ARTG Registration Step by Step RegDesk Medical Device Regulations Tga The australian register of therapeutic goods (artg) is the central database of therapeutic. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Medical device manufacturers (including ivds) in australia need: Personalised medical devices (pmd) commencing: Therapeutic goods (medical devices) regulations 2002. To determine which medical device regulations and standards apply to your company and specific. Medical Device Regulations Tga.
From www.pdffiller.com
Fillable Online tga gov Australian regulatory guidelines for medical devices medical devices Medical Device Regulations Tga Food and drug administration (fda), the european. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Department of health and aged care. Appropriate conformity assessment procedures in place for the device,. Therapeutic goods (medical devices) regulations 2002. To determine which medical device regulations and standards apply to your company and specific type of device (risk. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidance on Auditing of Medical Device Applications RegDesk Medical Device Regulations Tga Therapeutic goods (medical devices) regulations 2002. Reduced scope of custom made. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Department of health and aged care. Food and drug administration (fda), the european. Medical device manufacturers (including ivds) in australia need:. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidelines on Inclusion Process for Medical Devices (Including IVD) RegDesk Medical Device Regulations Tga Regulatory changes for software based medical devices. The australian register of therapeutic goods (artg) is the central database of therapeutic. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Reduced scope of custom made. Reclassification of active medical devices for therapy. Medical Device Regulations Tga.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Regulations Tga Regulatory changes for software based medical devices. Medical device manufacturers (including ivds) in australia need: The australian register of therapeutic goods (artg) is the central database of therapeutic. Reduced scope of custom made. This blog compares the criteria for medical device security standards outlined by the u.s. Department of health and aged care. Food and drug administration (fda), the european.. Medical Device Regulations Tga.
From www.slideshare.net
TGA Presentation Potential reforms for the regulation of system and Medical Device Regulations Tga Appropriate conformity assessment procedures in place for the device,. Department of health and aged care. Reduced scope of custom made. Reclassification of active medical devices for therapy with a diagnostic function. This blog compares the criteria for medical device security standards outlined by the u.s. The australian register of therapeutic goods (artg) is the central database of therapeutic. Food and. Medical Device Regulations Tga.
From www.regdesk.co
TGA on Reclassification of Medicine Delivering Medical Device RegDesk Medical Device Regulations Tga Personalised medical devices (pmd) commencing: Reclassification of active medical devices for therapy with a diagnostic function. Medical device manufacturers (including ivds) in australia need: The australian register of therapeutic goods (artg) is the central database of therapeutic. Food and drug administration (fda), the european. Appropriate conformity assessment procedures in place for the device,. Therapeutic goods (medical devices) regulations 2002. Department. Medical Device Regulations Tga.
From www.artixio.com
FAQs Australia (TGA) Regulations for Medical Device Registration Medical Device Regulations Tga Therapeutic goods (medical devices) regulations 2002. Medical device manufacturers (including ivds) in australia need: Department of health and aged care. The australian register of therapeutic goods (artg) is the central database of therapeutic. Appropriate conformity assessment procedures in place for the device,. Food and drug administration (fda), the european. Personalised medical devices (pmd) commencing: Regulatory changes for software based medical. Medical Device Regulations Tga.
From www.regdesk.co
Personalised Medical Devices TGA Regulation Overview RegDesk Medical Device Regulations Tga Reduced scope of custom made. Food and drug administration (fda), the european. Therapeutic goods (medical devices) regulations 2002. 25 feb 2021(new inclusions) changes: To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Reclassification of active medical devices for therapy with a. Medical Device Regulations Tga.
From www.mastercontrol.com
Therapeutic Goods Administration (TGA) and Medical Device Regulations Medical Device Regulations Tga Therapeutic goods (medical devices) regulations 2002. This blog compares the criteria for medical device security standards outlined by the u.s. Appropriate conformity assessment procedures in place for the device,. Reduced scope of custom made. Regulatory changes for software based medical devices. Medical device manufacturers (including ivds) in australia need: Department of health and aged care. To determine which medical device. Medical Device Regulations Tga.
From www.regdesk.co
TGA Consultation on Amendments to Regulation of Personalized Medical Devices RegDesk Medical Device Regulations Tga Regulatory changes for software based medical devices. Medical device manufacturers (including ivds) in australia need: Appropriate conformity assessment procedures in place for the device,. Food and drug administration (fda), the european. Reclassification of active medical devices for therapy with a diagnostic function. 25 feb 2021(new inclusions) changes: 11.45 programmed or programmable medical device or software that is a medical device—classification. Medical Device Regulations Tga.
From pedorthics.org.au
TGA Changes to the Regulation of CustomMade Medical Devices Pedorthic Association of Australia Medical Device Regulations Tga To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Therapeutic goods (medical devices) regulations 2002. Regulatory changes for software based medical devices. Personalised medical devices (pmd) commencing: This blog compares the criteria for medical device security standards outlined by the u.s.. Medical Device Regulations Tga.
From www.regdesk.co
TGA Guidelines of Regulation of SoftwareBased Medical Devices RegDesk Medical Device Regulations Tga The australian register of therapeutic goods (artg) is the central database of therapeutic. Regulatory changes for software based medical devices. This blog compares the criteria for medical device security standards outlined by the u.s. Department of health and aged care. Therapeutic goods (medical devices) regulations 2002. 11.45 programmed or programmable medical device or software that is a medical device—classification rules. Medical Device Regulations Tga.
From www.healthindustryhub.com.au
TGA unveils plan to transform medical device audits amidst global regulatory shifts Health Medical Device Regulations Tga Appropriate conformity assessment procedures in place for the device,. Food and drug administration (fda), the european. Therapeutic goods (medical devices) regulations 2002. 25 feb 2021(new inclusions) changes: Personalised medical devices (pmd) commencing: Reduced scope of custom made. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Regulatory changes for software based medical devices. Medical device. Medical Device Regulations Tga.
From www.r2pharma.com.au
TGA Medical Device Approval Regulations & Process Medical Device Regulations Tga 25 feb 2021(new inclusions) changes: This blog compares the criteria for medical device security standards outlined by the u.s. Department of health and aged care. Personalised medical devices (pmd) commencing: Food and drug administration (fda), the european. Regulatory changes for software based medical devices. Therapeutic goods (medical devices) regulations 2002. To determine which medical device regulations and standards apply to. Medical Device Regulations Tga.
From dokumen.tips
(PDF) Medical Devices Registration Guideline National Health€¦ · TGA) in order to harmonized Medical Device Regulations Tga Food and drug administration (fda), the european. Personalised medical devices (pmd) commencing: 11.45 programmed or programmable medical device or software that is a medical device—classification rules To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. Appropriate conformity assessment procedures in place. Medical Device Regulations Tga.
From www.regdesk.co
TGA Notice on CustomMade Medical Devices RegDesk Medical Device Regulations Tga This blog compares the criteria for medical device security standards outlined by the u.s. Reclassification of active medical devices for therapy with a diagnostic function. The australian register of therapeutic goods (artg) is the central database of therapeutic. Appropriate conformity assessment procedures in place for the device,. Department of health and aged care. Reduced scope of custom made. Medical device. Medical Device Regulations Tga.
From 4easyreg.com
Digital Medical Devices Regulation TGA Approach Medical Device Regulations Tga Regulatory changes for software based medical devices. The australian register of therapeutic goods (artg) is the central database of therapeutic. To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. 11.45 programmed or programmable medical device or software that is a medical. Medical Device Regulations Tga.
From www.tga.gov.au
Including IVD medical devices in the ARTG Therapeutic Goods Administration (TGA) Medical Device Regulations Tga Therapeutic goods (medical devices) regulations 2002. 11.45 programmed or programmable medical device or software that is a medical device—classification rules Personalised medical devices (pmd) commencing: Reduced scope of custom made. Reclassification of active medical devices for therapy with a diagnostic function. Medical device manufacturers (including ivds) in australia need: Regulatory changes for software based medical devices. Food and drug administration. Medical Device Regulations Tga.
From dokumen.tips
(PDF) EU Medical Device Regulation Implications for the TGA and DOKUMEN.TIPS Medical Device Regulations Tga Regulatory changes for software based medical devices. This blog compares the criteria for medical device security standards outlined by the u.s. 25 feb 2021(new inclusions) changes: Therapeutic goods (medical devices) regulations 2002. The australian register of therapeutic goods (artg) is the central database of therapeutic. Medical device manufacturers (including ivds) in australia need: Reduced scope of custom made. Appropriate conformity. Medical Device Regulations Tga.
From www.presentationeze.com
TGA Medical Device Classification Rule 5 Special RulesPresentationEZE Medical Device Regulations Tga Regulatory changes for software based medical devices. Reduced scope of custom made. 11.45 programmed or programmable medical device or software that is a medical device—classification rules To determine which medical device regulations and standards apply to your company and specific type of device (risk class), you will need to consult with the relevant competent. 25 feb 2021(new inclusions) changes: This. Medical Device Regulations Tga.