Titration Experiment Burette . Titrating sodium hydroxide with hydrochloric acid. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. A standard solution or titrant is placed in the. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a common laboratory technique for determining the concentration of a solute. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is a quantitative technique that uses a solution of known concentration to react completely with.
from mmerevise.co.uk
Titration is a common laboratory technique for determining the concentration of a solute. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is a quantitative technique that uses a solution of known concentration to react completely with. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. You have to decide if this experiment is suitable to use. A standard solution or titrant is placed in the.
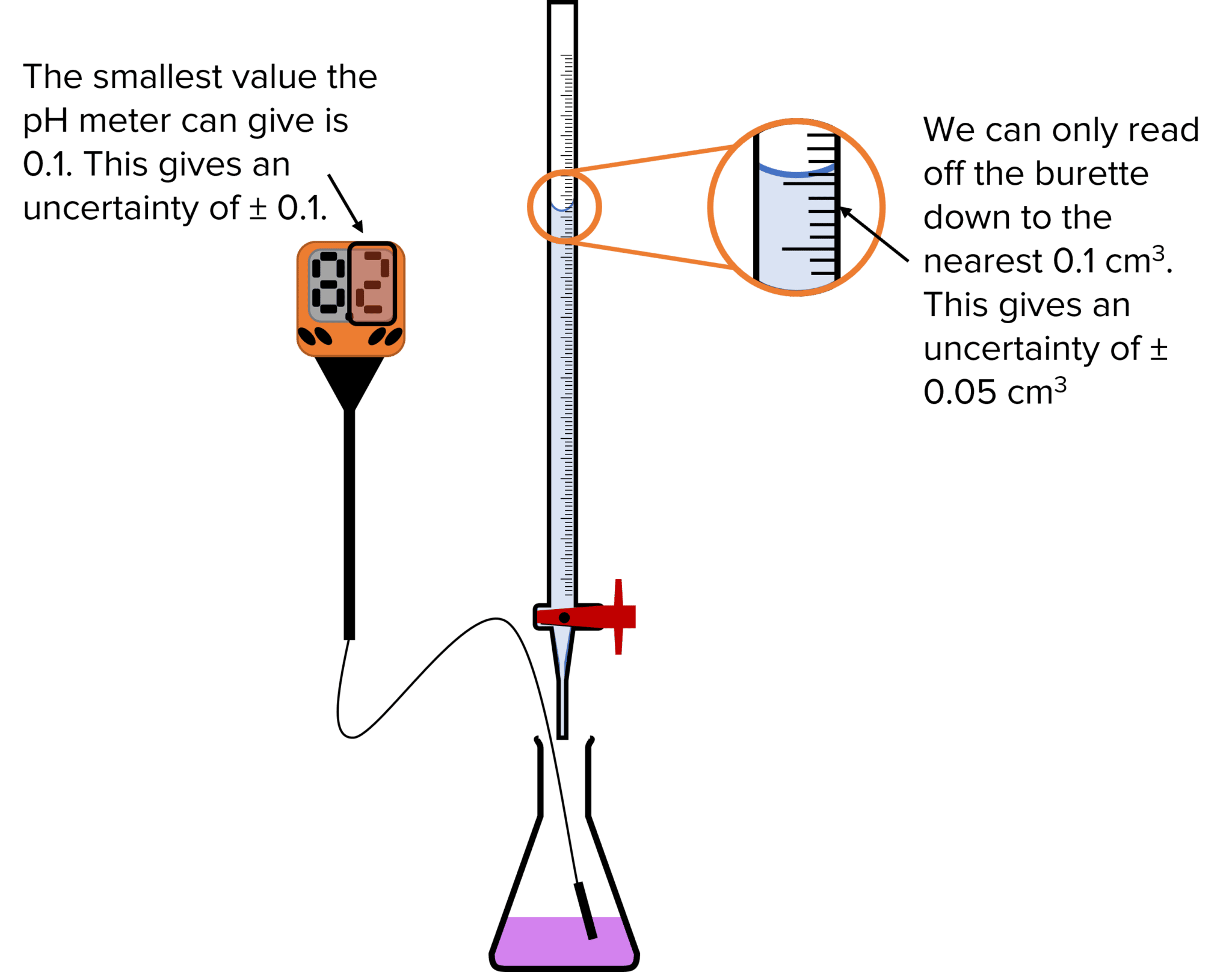
Titrations and Uncertainties MME
Titration Experiment Burette Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titrating sodium hydroxide with hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. A standard solution or titrant is placed in the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. You have to decide if this experiment is suitable to use.
From www.numerade.com
SOLVED The student repeated the titration experiment three times. The readings on the burette Titration Experiment Burette Titration is a common laboratory technique for determining the concentration of a solute. You have to decide if this experiment is suitable to use. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for. Titration Experiment Burette.
From www.alamy.com
Chemistry student in an undergraduate teaching laboratory carrying out a titration experiment. A Titration Experiment Burette You have to decide if this experiment is suitable to use. Titrating sodium hydroxide with hydrochloric acid. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a common laboratory technique for. Titration Experiment Burette.
From www.dreamstime.com
Titration stock photo. Image of biology, analyzing, burette 231791018 Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrating sodium hydroxide with hydrochloric acid. A standard solution or titrant is placed in the. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is a quantitative technique that uses a solution of known. Titration Experiment Burette.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. You have to decide if this experiment is suitable to use. Learn to use a burette for titration and other experiments a burette (or buret) is. Titration Experiment Burette.
From ar.inspiredpencil.com
Burette Titration Titration Experiment Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titrating sodium hydroxide with hydrochloric acid. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab. Titration Experiment Burette.
From www.vrogue.co
Titration Apparatus vrogue.co Titration Experiment Burette Titrating sodium hydroxide with hydrochloric acid. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. You have to decide if this experiment is suitable to use. A standard solution or titrant is placed in the. Titration is a quantitative technique that uses a solution of known. Titration Experiment Burette.
From acidsandbasesfordummieschem.weebly.com
Titration Acid and Bases for Dummies! Titration Experiment Burette A standard solution or titrant is placed in the. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. You have to decide if this experiment is suitable. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to carry out an acidbase Titration Experiment Burette You have to decide if this experiment is suitable to use. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. Titration Experiment Burette.
From www.etsy.com
Titration Equipment Set Complete Single Buret Burete Assembly Etsy UK Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution. Titration Experiment Burette.
From www.dreamstime.com
Redox Titration with Burette and Pipette Stock Vector Illustration of measurement, laboratory Titration Experiment Burette A standard solution or titrant is placed in the. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. Titration is a common laboratory technique for. Titration Experiment Burette.
From techblog.ctgclean.com
Chemistry What is Titration? CTG Clean Titration Experiment Burette Titration is a quantitative technique that uses a solution of known concentration to react completely with. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. A standard solution or titrant is placed in the. A buret is primarily used for titration to determine the concentration of an unknown solution by. Titration Experiment Burette.
From www.alamy.com
A burette used for titrations Stock Photo Alamy Titration Experiment Burette A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. Titrating. Titration Experiment Burette.
From pixels.com
Titration Experiment Photograph by Andrew Lambert Photography/science Photo Library Titration Experiment Burette You have to decide if this experiment is suitable to use. Titrating sodium hydroxide with hydrochloric acid. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is a quantitative technique that uses a solution of known concentration to react completely with. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Titration Experiment Burette.
From stock.adobe.com
Titration Experiment (Burette and Flask for Titration) simple minimalist isolated in white Titration Experiment Burette A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. A standard solution or titrant is placed in the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to. Titration Experiment Burette.
From stock.adobe.com
Laboratory experiment of acid base titration with glass burette and Erlenmeyer flask and text Titration Experiment Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A standard solution or titrant is placed in the. Titration is a common laboratory technique for determining the concentration of a solute. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Learn to use a. Titration Experiment Burette.
From www.canfortlab.com
Marble titration stand, burette stand Titration Experiment Burette Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. You have to decide if this experiment is suitable to use. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Titration is a common laboratory technique for determining the concentration. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student adding an alkaline solution from a burette to a solution of citric Titration Experiment Burette You have to decide if this experiment is suitable to use. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a common laboratory technique for determining the concentration of a solute. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals.. Titration Experiment Burette.
From www.youtube.com
Titration Step 2 Preparing the burette YouTube Titration Experiment Burette You have to decide if this experiment is suitable to use. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for dispensing fluids into. Titrating sodium hydroxide with hydrochloric acid. They. Titration Experiment Burette.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Experiment Burette A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. A standard solution or titrant is placed in the. Titration is a quantitative technique that uses a solution of known concentration to react completely with. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers. Titration Experiment Burette.
From www.amazon.com
Titration Equipment Set Complete Single Buret Burete Assembly with 100 mL Acrylic Buret 8 x 5 Titration Experiment Burette You have to decide if this experiment is suitable to use. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Learn to use a burette for titration and other. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student taking a volume reading from a burette during an acidbase Titration Experiment Burette A standard solution or titrant is placed in the. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a common laboratory. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to carry out an acidbase Titration Experiment Burette Titrating sodium hydroxide with hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Learn to use a burette for titration and other experiments a burette (or buret) is a handy lab tool for. Titration Experiment Burette.
From science.wonderhowto.com
How to Perform Titration with a Burette in the Chemistry Lab « Science Experiments WonderHowTo Titration Experiment Burette A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. A standard solution or titrant is placed in the. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Learn to use a burette for titration and other experiments a burette (or buret). Titration Experiment Burette.
From www.pngwing.com
Titration Beaker, Buret s, glass, angle, laboratory png PNGWing Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrating sodium hydroxide with hydrochloric acid. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use. A buret is primarily used for titration to determine. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to carry out a titration Titration Experiment Burette Titration is a common laboratory technique for determining the concentration of a solute. Titration is a quantitative technique that uses a solution of known concentration to react completely with. A standard solution or titrant is placed in the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Learn to use. Titration Experiment Burette.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to carry out an acidbase Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration. Titration Experiment Burette.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004_titrationapparatussetup Titration Experiment Burette Titration is a common laboratory technique for determining the concentration of a solute. Titrating sodium hydroxide with hydrochloric acid. Titration is a quantitative technique that uses a solution of known concentration to react completely with. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. A standard solution or titrant is placed in the. Learn. Titration Experiment Burette.
From pixels.com
Titration Experiment Photograph by Titration Experiment Burette You have to decide if this experiment is suitable to use. A standard solution or titrant is placed in the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is a common laboratory technique for determining the concentration of a solute. Titrating sodium hydroxide with hydrochloric acid. To perform. Titration Experiment Burette.
From www.istockphoto.com
Redox Titration With Burette And Pipette Stock Illustration Download Image Now Titration Titration Experiment Burette To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrating sodium hydroxide with hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. Titration is a. Titration Experiment Burette.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a quantitative technique that uses a solution of known concentration to react. Titration Experiment Burette.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Experiment Burette In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrating sodium hydroxide with hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. You have to decide if this experiment is suitable to use. A buret is primarily used for titration to determine the concentration. Titration Experiment Burette.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Experiment Burette A standard solution or titrant is placed in the. Titrating sodium hydroxide with hydrochloric acid. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a common laboratory technique for determining the concentration of a solute. Titration is a quantitative technique that uses a solution of known. Titration Experiment Burette.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Experiment Burette Titration is a common laboratory technique for determining the concentration of a solute. Titration is a quantitative technique that uses a solution of known concentration to react completely with. To perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a measured. Learn to use a burette for titration and other experiments a burette. Titration Experiment Burette.
From scientificservices.eu
Titration Burette UseScience Titration Experiment Burette They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is a common laboratory technique for determining the concentration of a solute. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Learn to use a burette for titration and other experiments a burette (or buret) is a. Titration Experiment Burette.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer flask. Pipette and Titration Experiment Burette Titration is a common laboratory technique for determining the concentration of a solute. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to. Titration Experiment Burette.