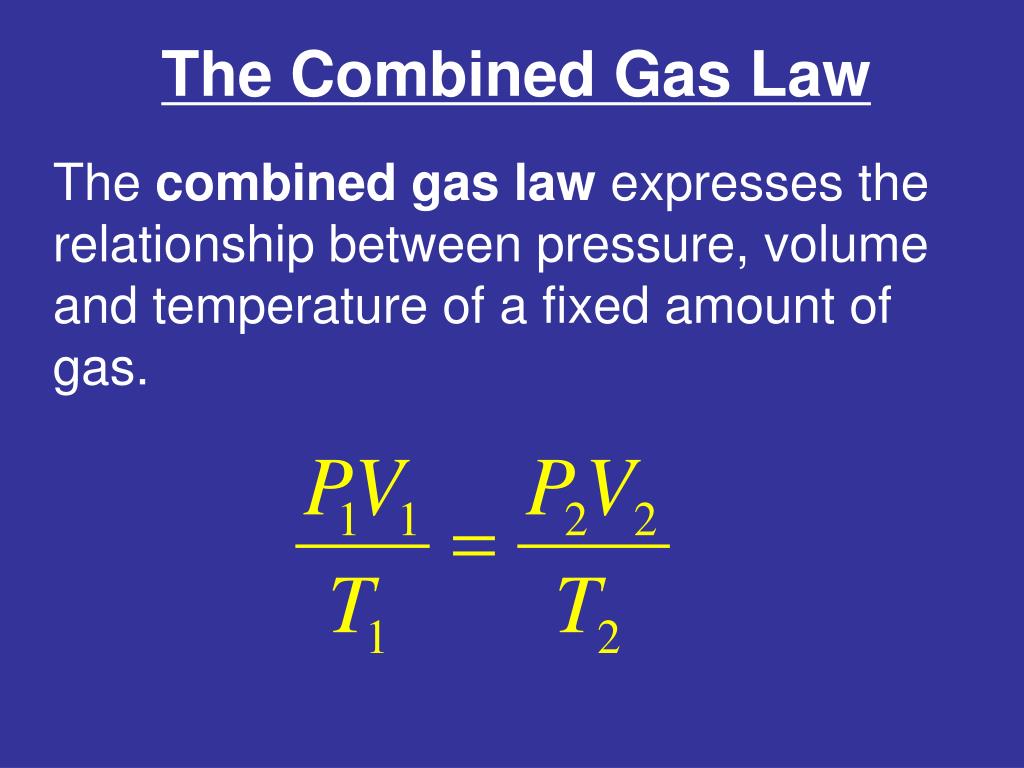
Does Combined Gas Law Have To Be In Kelvin . find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. For the other variables any relevant unit may be used, but the units. as with the ideal gas law, the temperature must be expressed in kelvin. P total is the combined pressure of the dry gas and the water vapor. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). The constant k is a true constant if the number of moles of the gas doesn't change. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. P 1 v 1 / t 1 = p 2 v 2 / t 2. it is important to recognize the p total is the 98.0 value. First convert 25.0 °c to the kelvin scale. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. Another common formula for the combined gas law relates before and after conditions of a gas:
from www.slideserve.com
it is important to recognize the p total is the 98.0 value. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. P total is the combined pressure of the dry gas and the water vapor. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. Another common formula for the combined gas law relates before and after conditions of a gas: For the other variables any relevant unit may be used, but the units. P 1 v 1 / t 1 = p 2 v 2 / t 2. as with the ideal gas law, the temperature must be expressed in kelvin. First convert 25.0 °c to the kelvin scale. use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\).
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint
Does Combined Gas Law Have To Be In Kelvin again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. For the other variables any relevant unit may be used, but the units. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. P total is the combined pressure of the dry gas and the water vapor. as with the ideal gas law, the temperature must be expressed in kelvin. First convert 25.0 °c to the kelvin scale. P 1 v 1 / t 1 = p 2 v 2 / t 2. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. Another common formula for the combined gas law relates before and after conditions of a gas: for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. The constant k is a true constant if the number of moles of the gas doesn't change. use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). it is important to recognize the p total is the 98.0 value.
From slideplayer.com
Gas Laws Section ppt download Does Combined Gas Law Have To Be In Kelvin Another common formula for the combined gas law relates before and after conditions of a gas: Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. find the volume of. Does Combined Gas Law Have To Be In Kelvin.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Does Combined Gas Law Have To Be In Kelvin for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. P total is the combined pressure of the dry gas and the water vapor. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in. Does Combined Gas Law Have To Be In Kelvin.
From exotnbeyi.blob.core.windows.net
Combined Gas Law Ppt at Dennis House blog Does Combined Gas Law Have To Be In Kelvin P 1 v 1 / t 1 = p 2 v 2 / t 2. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. it. Does Combined Gas Law Have To Be In Kelvin.
From www.sliderbase.com
Gas Laws Presentation Chemistry Does Combined Gas Law Have To Be In Kelvin P 1 v 1 / t 1 = p 2 v 2 / t 2. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0. Does Combined Gas Law Have To Be In Kelvin.
From slideplayer.com
The Gas Laws Mathematical relationships between volume, temperature Does Combined Gas Law Have To Be In Kelvin use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). For the other variables any relevant unit may be used, but the units. The constant k is a true constant if the number of moles of the gas doesn't change. P total is the combined pressure of the dry gas and the water vapor. . Does Combined Gas Law Have To Be In Kelvin.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Does Combined Gas Law Have To Be In Kelvin find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. P total is the combined pressure of the dry gas and the water vapor. The constant k is a true constant if the number of moles of the gas doesn't change. use. Does Combined Gas Law Have To Be In Kelvin.
From mmerevise.co.uk
The Ideal Gas Equation MME Does Combined Gas Law Have To Be In Kelvin Another common formula for the combined gas law relates before and after conditions of a gas: First convert 25.0 °c to the kelvin scale. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. P total is the combined pressure of the dry gas and. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT The Gas Laws PowerPoint Presentation, free download ID3358137 Does Combined Gas Law Have To Be In Kelvin Another common formula for the combined gas law relates before and after conditions of a gas: First convert 25.0 °c to the kelvin scale. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the. Does Combined Gas Law Have To Be In Kelvin.
From www.showme.com
When to use combined or ideal gas law ShowMe Does Combined Gas Law Have To Be In Kelvin as with the ideal gas law, the temperature must be expressed in kelvin. For the other variables any relevant unit may be used, but the units. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. it is important to recognize the p total is the 98.0 value. find the volume. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Combined Gas Laws and Dalton’s Law of Partial Pressures Does Combined Gas Law Have To Be In Kelvin again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. as with the ideal gas law, the temperature must be expressed in kelvin. First convert 25.0 °c to the kelvin scale. find the volume of a gas at 760.0 mm hg pressure and. Does Combined Gas Law Have To Be In Kelvin.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Does Combined Gas Law Have To Be In Kelvin use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). The constant k is a true constant if the number of moles of the gas doesn't change. For the other variables any relevant unit may be used, but the units. find the volume of a gas at 760.0 mm hg pressure and 273 k. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Does Combined Gas Law Have To Be In Kelvin For the other variables any relevant unit may be used, but the units. The constant k is a true constant if the number of moles of the gas doesn't change. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. First convert 25.0 °c to the kelvin scale. find the volume of a. Does Combined Gas Law Have To Be In Kelvin.
From sciencenotes.org
Ideal Gas Law Formula and Examples Does Combined Gas Law Have To Be In Kelvin again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. for all gas law problems it is. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Does Combined Gas Law Have To Be In Kelvin For the other variables any relevant unit may be used, but the units. use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). it is important to recognize the p total is the 98.0 value. Another common formula for the combined gas law relates before and after conditions of a gas: P 1 v. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID2715657 Does Combined Gas Law Have To Be In Kelvin For the other variables any relevant unit may be used, but the units. for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. it is important to recognize the p total is the 98.0 value. find the volume of a gas at 760.0 mm hg. Does Combined Gas Law Have To Be In Kelvin.
From materialfullagonises.z13.web.core.windows.net
Combined Gas Law Explained Does Combined Gas Law Have To Be In Kelvin as with the ideal gas law, the temperature must be expressed in kelvin. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. P 1 v. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Properties of Gases & Gas Laws PowerPoint Presentation, free Does Combined Gas Law Have To Be In Kelvin First convert 25.0 °c to the kelvin scale. it is important to recognize the p total is the 98.0 value. P 1 v 1 / t 1 = p 2 v 2 / t 2. P total is the combined pressure of the dry gas and the water vapor. again, the usual warnings apply about how to solve. Does Combined Gas Law Have To Be In Kelvin.
From learningschoolballaurixh.z4.web.core.windows.net
Combined Gas Law Explanation Does Combined Gas Law Have To Be In Kelvin Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. it is important to recognize the p total is the 98.0 value. For the other variables any relevant. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID5015618 Does Combined Gas Law Have To Be In Kelvin P total is the combined pressure of the dry gas and the water vapor. First convert 25.0 °c to the kelvin scale. as with the ideal gas law, the temperature must be expressed in kelvin. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the. Does Combined Gas Law Have To Be In Kelvin.
From learningravnihym.z21.web.core.windows.net
Combined Gas Law Explained Does Combined Gas Law Have To Be In Kelvin The constant k is a true constant if the number of moles of the gas doesn't change. it is important to recognize the p total is the 98.0 value. as with the ideal gas law, the temperature must be expressed in kelvin. P total is the combined pressure of the dry gas and the water vapor. For the. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID3099616 Does Combined Gas Law Have To Be In Kelvin again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. Another common formula for the combined gas law. Does Combined Gas Law Have To Be In Kelvin.
From owlcation.com
The Theories and Behavior of Gas Owlcation Does Combined Gas Law Have To Be In Kelvin for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. For the other variables any relevant unit may be used, but the units. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Law and Gas Behavior PowerPoint Presentation ID3183351 Does Combined Gas Law Have To Be In Kelvin First convert 25.0 °c to the kelvin scale. use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). as with the ideal gas law, the temperature must be expressed in kelvin. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Does Combined Gas Law Have To Be In Kelvin it is important to recognize the p total is the 98.0 value. For the other variables any relevant unit may be used, but the units. P total is the combined pressure of the dry gas and the water vapor. Another common formula for the combined gas law relates before and after conditions of a gas: First convert 25.0 °c. Does Combined Gas Law Have To Be In Kelvin.
From printableslashasylumt7.z22.web.core.windows.net
Combined Gas Law Explained Does Combined Gas Law Have To Be In Kelvin P total is the combined pressure of the dry gas and the water vapor. Another common formula for the combined gas law relates before and after conditions of a gas: P 1 v 1 / t 1 = p 2 v 2 / t 2. as with the ideal gas law, the temperature must be expressed in kelvin. The. Does Combined Gas Law Have To Be In Kelvin.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Combined Gas Law Have To Be In Kelvin as with the ideal gas law, the temperature must be expressed in kelvin. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. First convert 25.0 °c to the kelvin scale. for all gas law problems it is necessary to work. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Does Combined Gas Law Have To Be In Kelvin Another common formula for the combined gas law relates before and after conditions of a gas: First convert 25.0 °c to the kelvin scale. it is important to recognize the p total is the 98.0 value. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Laws and Nature of Gases PowerPoint Presentation, free Does Combined Gas Law Have To Be In Kelvin as with the ideal gas law, the temperature must be expressed in kelvin. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. The constant k is a true constant if the number of moles of the gas doesn't change. For the. Does Combined Gas Law Have To Be In Kelvin.
From learningravnihym.z21.web.core.windows.net
Combined Gas Law Explanation Does Combined Gas Law Have To Be In Kelvin use the combined gas law to solve for the unknown volume \(\left( v_2 \right)\). Another common formula for the combined gas law relates before and after conditions of a gas: For the other variables any relevant unit may be used, but the units. for all gas law problems it is necessary to work in the kelvin scale because. Does Combined Gas Law Have To Be In Kelvin.
From printablenensikazizn.z21.web.core.windows.net
Combined Gas Law Explained Does Combined Gas Law Have To Be In Kelvin Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. The constant k is a true constant if the number of moles of the gas doesn't change. as with the ideal gas law, the temperature must be expressed in kelvin. find the volume of a gas at 760.0 mm hg pressure and. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Chapter 11 Properties of Gases PowerPoint Presentation, free Does Combined Gas Law Have To Be In Kelvin First convert 25.0 °c to the kelvin scale. P 1 v 1 / t 1 = p 2 v 2 / t 2. The constant k is a true constant if the number of moles of the gas doesn't change. Another common formula for the combined gas law relates before and after conditions of a gas: for all gas. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4327408 Does Combined Gas Law Have To Be In Kelvin as with the ideal gas law, the temperature must be expressed in kelvin. find the volume of a gas at 760.0 mm hg pressure and 273 k when 2.00 liters is collected at 745.0 mm hg and 25.0 °c. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. again, the. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT Gas Relationships PowerPoint Presentation, free download ID2707799 Does Combined Gas Law Have To Be In Kelvin for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. P total is the combined pressure of the dry gas and the water vapor. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. First convert 25.0 °c to the kelvin. Does Combined Gas Law Have To Be In Kelvin.
From printableneteb3.z21.web.core.windows.net
Combined Gas Law Explained Does Combined Gas Law Have To Be In Kelvin for all gas law problems it is necessary to work in the kelvin scale because temperature is in the denominator in the. Where p = pressure, v = volume, t = absolute temperature (kelvin), and k = constant. P total is the combined pressure of the dry gas and the water vapor. it is important to recognize the. Does Combined Gas Law Have To Be In Kelvin.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Does Combined Gas Law Have To Be In Kelvin Another common formula for the combined gas law relates before and after conditions of a gas: The constant k is a true constant if the number of moles of the gas doesn't change. again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator),. as. Does Combined Gas Law Have To Be In Kelvin.