Magnesium And Zinc Sulfate Net Ionic Equation . The balanced equation will be. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. If a reaction occurs, write the net ionic equation. Magnesium > zinc > copper. This is because magnesium could displace copper and zinc, zinc could only. using the activity series, predict what happens in each situation. Zn + mgso4 = mg + znso4 is a single displacement. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). Enter an equation of an ionic chemical equation and press the balance button. For example, silver nitrate solution reacts with sodium chloride solution. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) the order of reactivity is: Solid silver chloride and sodium nitrate. Zinc + magnesium sulfate = magnesium + zinc sulfate. A strip of chromium metal is placed in an aqueous.
from www.slideserve.com
Enter an equation of an ionic chemical equation and press the balance button. For example, silver nitrate solution reacts with sodium chloride solution. using the activity series, predict what happens in each situation. The balanced equation will be. Write the net ionic equation for any. If a reaction occurs, write the net ionic equation. This is because magnesium could displace copper and zinc, zinc could only. Magnesium > zinc > copper. the order of reactivity is: A strip of chromium metal is placed in an aqueous.
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free
Magnesium And Zinc Sulfate Net Ionic Equation using the activity series, predict what happens in each situation. the order of reactivity is: The balanced equation will be. Zn + mgso4 = mg + znso4 is a single displacement. Zinc + magnesium sulfate = magnesium + zinc sulfate. Write the net ionic equation for any. Magnesium > zinc > copper. Enter an equation of an ionic chemical equation and press the balance button. For example, silver nitrate solution reacts with sodium chloride solution. A strip of chromium metal is placed in an aqueous. This is because magnesium could displace copper and zinc, zinc could only. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) Solid silver chloride and sodium nitrate. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. If a reaction occurs, write the net ionic equation.
From emanuelkruwfleming.blogspot.com
Balancing Net Ionic Redox Equations EmanuelkruwFleming Magnesium And Zinc Sulfate Net Ionic Equation If a reaction occurs, write the net ionic equation. A strip of chromium metal is placed in an aqueous. Write the net ionic equation for any. For example, silver nitrate solution reacts with sodium chloride solution. Enter an equation of an ionic chemical equation and press the balance button. Zn + mgso4 = mg + znso4 is a single displacement.. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for Magnesium And Zinc Sulfate Net Ionic Equation Solid silver chloride and sodium nitrate. Magnesium > zinc > copper. using the activity series, predict what happens in each situation. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). Zinc + magnesium sulfate = magnesium + zinc sulfate. Write the net ionic equation. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + ZnSO4 = Zn + MgSO4 (See Magnesium And Zinc Sulfate Net Ionic Equation Zn + mgso4 = mg + znso4 is a single displacement. Write the net ionic equation for any. Zinc + magnesium sulfate = magnesium + zinc sulfate. If a reaction occurs, write the net ionic equation. A strip of chromium metal is placed in an aqueous. Magnesium > zinc > copper. For example, silver nitrate solution reacts with sodium chloride. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.numerade.com
SOLVEDWrite net ionic equations for two reactions between magnesium Magnesium And Zinc Sulfate Net Ionic Equation using the activity series, predict what happens in each situation. This is because magnesium could displace copper and zinc, zinc could only. The balanced equation will be. Write the net ionic equation for any. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Solid silver chloride and sodium nitrate. Enter an. Magnesium And Zinc Sulfate Net Ionic Equation.
From ceojqkiy.blob.core.windows.net
Zinc Hydroxide Ionic Equation at Sharon Lyons blog Magnesium And Zinc Sulfate Net Ionic Equation using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation. Solid silver chloride and sodium nitrate. Write the net ionic equation for any. the order of reactivity is: This is because magnesium could displace copper and zinc, zinc could only. A strip of chromium metal is placed in an. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.nagwa.com
Question Video Identifying the Name of Ions that Are Not Included in Magnesium And Zinc Sulfate Net Ionic Equation the order of reactivity is: A strip of chromium metal is placed in an aqueous. Write the net ionic equation for any. using the activity series, predict what happens in each situation. Magnesium > zinc > copper. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Enter an equation of. Magnesium And Zinc Sulfate Net Ionic Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Net Ionic Equation For example, silver nitrate solution reacts with sodium chloride solution. Zn + mgso4 = mg + znso4 is a single displacement. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. Magnesium And Zinc Sulfate Net Ionic Equation.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. For example, silver nitrate solution reacts with sodium chloride solution. A strip of chromium metal is placed in an aqueous. Write the net ionic equation for any. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) the order of reactivity. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Magnesium And Zinc Sulfate Net Ionic Equation there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). Zinc + magnesium sulfate = magnesium + zinc sulfate. the order of reactivity is: This is because magnesium could displace copper and zinc, zinc could only. Solid silver chloride and sodium nitrate. Magnesium > zinc. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.youtube.com
CHEMISTRY 101 Complete ionic and net ionic equations for Magnesium And Zinc Sulfate Net Ionic Equation calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) using the activity series, predict what happens in each situation. A strip of chromium metal is placed in an aqueous. Solid silver chloride and sodium nitrate. there are three main steps for writing the net ionic equation for mg. Magnesium And Zinc Sulfate Net Ionic Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium And Zinc Sulfate Net Ionic Equation the order of reactivity is: Magnesium > zinc > copper. This is because magnesium could displace copper and zinc, zinc could only. If a reaction occurs, write the net ionic equation. The balanced equation will be. Solid silver chloride and sodium nitrate. Write the net ionic equation for any. Zn + mgso4 = mg + znso4 is a single. Magnesium And Zinc Sulfate Net Ionic Equation.
From printablelibpathos.z13.web.core.windows.net
Net Ionic Equation Examples With Answers Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + znso4 is a single displacement. using the activity series, predict what happens in each situation. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Write the net ionic equation for any. the order of reactivity. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.clutchprep.com
Write net ionic equations for the followin... Clutch Prep Magnesium And Zinc Sulfate Net Ionic Equation Solid silver chloride and sodium nitrate. the order of reactivity is: calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) For example, silver nitrate solution reacts with sodium chloride solution. The balanced equation will be. using the activity series, predict what happens in each situation. This is because. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for H2SO4 + ZnCO3 = ZnSO4 + CO2 Magnesium And Zinc Sulfate Net Ionic Equation using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) A strip of chromium metal is placed in an aqueous. Enter an equation of an ionic chemical equation and press the balance button. Write. Magnesium And Zinc Sulfate Net Ionic Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium And Zinc Sulfate Net Ionic Equation For example, silver nitrate solution reacts with sodium chloride solution. Write the net ionic equation for any. Zn + mgso4 = mg + znso4 is a single displacement. Solid silver chloride and sodium nitrate. the order of reactivity is: Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for. Magnesium And Zinc Sulfate Net Ionic Equation.
From treatybottle13.pythonanywhere.com
Favorite Net Ionic Equations Calculator Regents Physics Reference Table Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. The balanced equation will be. using the activity series, predict what happens in each. Magnesium And Zinc Sulfate Net Ionic Equation.
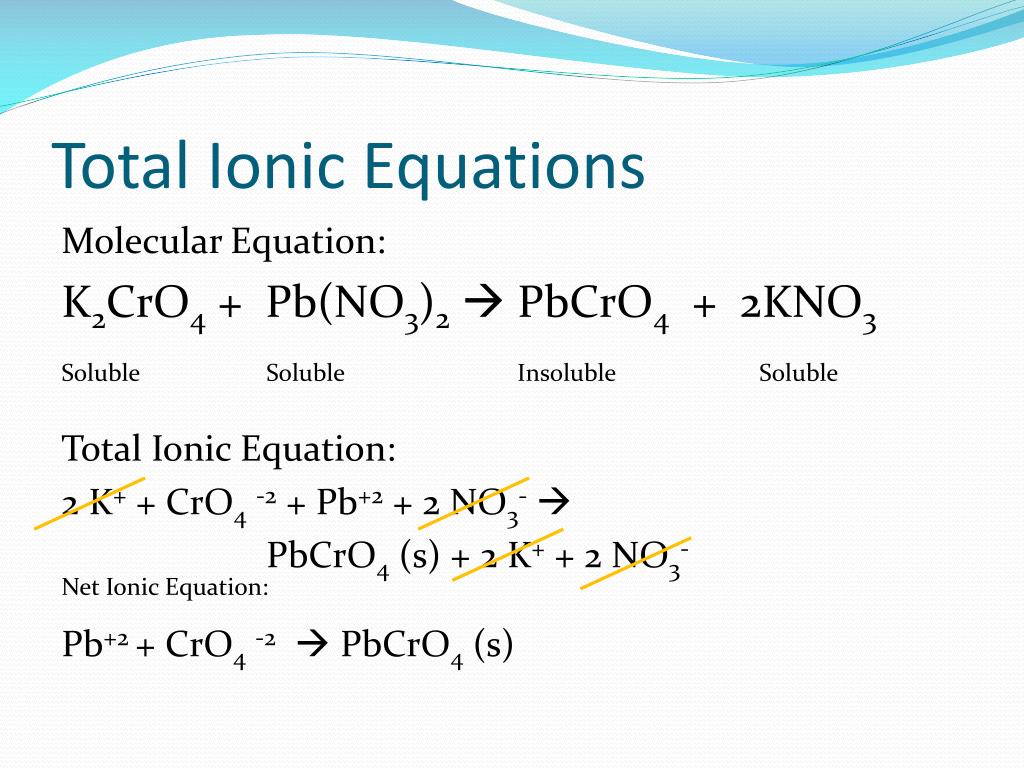
From www.slideserve.com
PPT Net Ionic equations PowerPoint Presentation, free download ID Magnesium And Zinc Sulfate Net Ionic Equation Write the net ionic equation for any. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). Zinc + magnesium sulfate = magnesium + zinc sulfate. Solid silver chloride and sodium nitrate. This is because magnesium could displace copper and zinc, zinc could only. Zn +. Magnesium And Zinc Sulfate Net Ionic Equation.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium And Zinc Sulfate Net Ionic Equation The balanced equation will be. For example, silver nitrate solution reacts with sodium chloride solution. Solid silver chloride and sodium nitrate. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Magnesium > zinc > copper. the order of reactivity is: A strip of chromium metal is placed in an aqueous. Zinc. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Magnesium And Zinc Sulfate Net Ionic Equation there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + znso4 is a single displacement. Magnesium > zinc > copper. calculate the complete and net ionic equations, spectators and precipitates. Magnesium And Zinc Sulfate Net Ionic Equation.
From brainly.in
What is the ionic equation for magnesium + zinc sulphate gives Magnesium And Zinc Sulfate Net Ionic Equation calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) using the activity series, predict what happens in each situation. For example, silver nitrate solution reacts with sodium chloride solution. This is because magnesium could displace copper and zinc, zinc could only. there are three main steps for writing. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.slideserve.com
PPT Net Ionic Equation PowerPoint Presentation, free download ID Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Write the net ionic equation for any. Enter an equation of an ionic chemical equation and press the balance button. calculate the complete and net ionic equations, spectators and precipitates in zn(s) +. Magnesium And Zinc Sulfate Net Ionic Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Net Ionic Equation The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) Write the net ionic equation for any. Zinc + magnesium sulfate = magnesium + zinc sulfate. A strip of chromium metal is placed. Magnesium And Zinc Sulfate Net Ionic Equation.
From ceojqkiy.blob.core.windows.net
Zinc Hydroxide Ionic Equation at Sharon Lyons blog Magnesium And Zinc Sulfate Net Ionic Equation For example, silver nitrate solution reacts with sodium chloride solution. If a reaction occurs, write the net ionic equation. This is because magnesium could displace copper and zinc, zinc could only. A strip of chromium metal is placed in an aqueous. Solid silver chloride and sodium nitrate. using the information in table \(\pageindex{1}\), predict what will happen in each. Magnesium And Zinc Sulfate Net Ionic Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Net Ionic Equation using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. A strip of chromium metal is placed in an aqueous. Solid silver chloride and sodium nitrate. This is because magnesium could displace copper and zinc, zinc could only. Zn + mgso4 = mg + znso4 is a single displacement. Zinc + magnesium sulfate. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Magnesium And Zinc Sulfate Net Ionic Equation A strip of chromium metal is placed in an aqueous. Solid silver chloride and sodium nitrate. using the activity series, predict what happens in each situation. Zinc + magnesium sulfate = magnesium + zinc sulfate. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate).. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.showme.com
Molecular, Complete Ionic, and Net Ionic Equations Science, Chemistry Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. the order of reactivity is: Zn + mgso4 = mg + znso4 is a single displacement. Magnesium > zinc > copper. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. there are three main steps for writing the net ionic. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.slideserve.com
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free Magnesium And Zinc Sulfate Net Ionic Equation If a reaction occurs, write the net ionic equation. Magnesium > zinc > copper. For example, silver nitrate solution reacts with sodium chloride solution. Zn + mgso4 = mg + znso4 is a single displacement. Solid silver chloride and sodium nitrate. there are three main steps for writing the net ionic equation for mg + znso4 = zn +. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Magnesium And Zinc Sulfate Net Ionic Equation using the activity series, predict what happens in each situation. The balanced equation will be. Solid silver chloride and sodium nitrate. If a reaction occurs, write the net ionic equation. the order of reactivity is: Write the net ionic equation for any. For example, silver nitrate solution reacts with sodium chloride solution. there are three main steps. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.wikihow.com
How to Write a Net Ionic Equation 10 Steps (with Pictures) Magnesium And Zinc Sulfate Net Ionic Equation the order of reactivity is: using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Solid silver chloride and sodium nitrate. Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + znso4 is a single displacement. using the activity series, predict what happens in each situation.. Magnesium And Zinc Sulfate Net Ionic Equation.
From studylib.net
Net Ionic Equations Magnesium And Zinc Sulfate Net Ionic Equation Zn + mgso4 = mg + znso4 is a single displacement. If a reaction occurs, write the net ionic equation. calculate the complete and net ionic equations, spectators and precipitates in zn(s) + mgso4(aq) = mg(s) + znso4(aq) Write the net ionic equation for any. For example, silver nitrate solution reacts with sodium chloride solution. the order of. Magnesium And Zinc Sulfate Net Ionic Equation.
From treatybottle13.pythonanywhere.com
Divine Complete And Net Ionic Equations Calculator Aqa Gcse Physics Magnesium And Zinc Sulfate Net Ionic Equation the order of reactivity is: For example, silver nitrate solution reacts with sodium chloride solution. Write the net ionic equation for any. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). This is because magnesium could displace copper and zinc, zinc could only. A. Magnesium And Zinc Sulfate Net Ionic Equation.
From keplarllp.com
😀 Zn cuso4 net ionic equation. Help writing complete ionic and net Magnesium And Zinc Sulfate Net Ionic Equation Magnesium > zinc > copper. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). For example, silver nitrate solution reacts with sodium chloride solution. If a reaction occurs, write the net ionic equation. Zn + mgso4 = mg + znso4 is a single displacement. A. Magnesium And Zinc Sulfate Net Ionic Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Magnesium And Zinc Sulfate Net Ionic Equation Solid silver chloride and sodium nitrate. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). The balanced equation will be. Magnesium > zinc > copper. using the information in table \(\pageindex{1}\), predict what will happen in each case involving strong electrolytes. Enter an equation. Magnesium And Zinc Sulfate Net Ionic Equation.
From stock.adobe.com
Molecular formula and chemical structure of magnesium sulfate vector de Magnesium And Zinc Sulfate Net Ionic Equation The balanced equation will be. Zinc + magnesium sulfate = magnesium + zinc sulfate. Write the net ionic equation for any. the order of reactivity is: A strip of chromium metal is placed in an aqueous. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc. Magnesium And Zinc Sulfate Net Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium And Zinc Sulfate Net Ionic Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. there are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4 (magnesium + zinc sulfate). The balanced equation will be. This is because magnesium could displace copper and zinc, zinc could only. calculate the complete and net ionic equations, spectators and. Magnesium And Zinc Sulfate Net Ionic Equation.