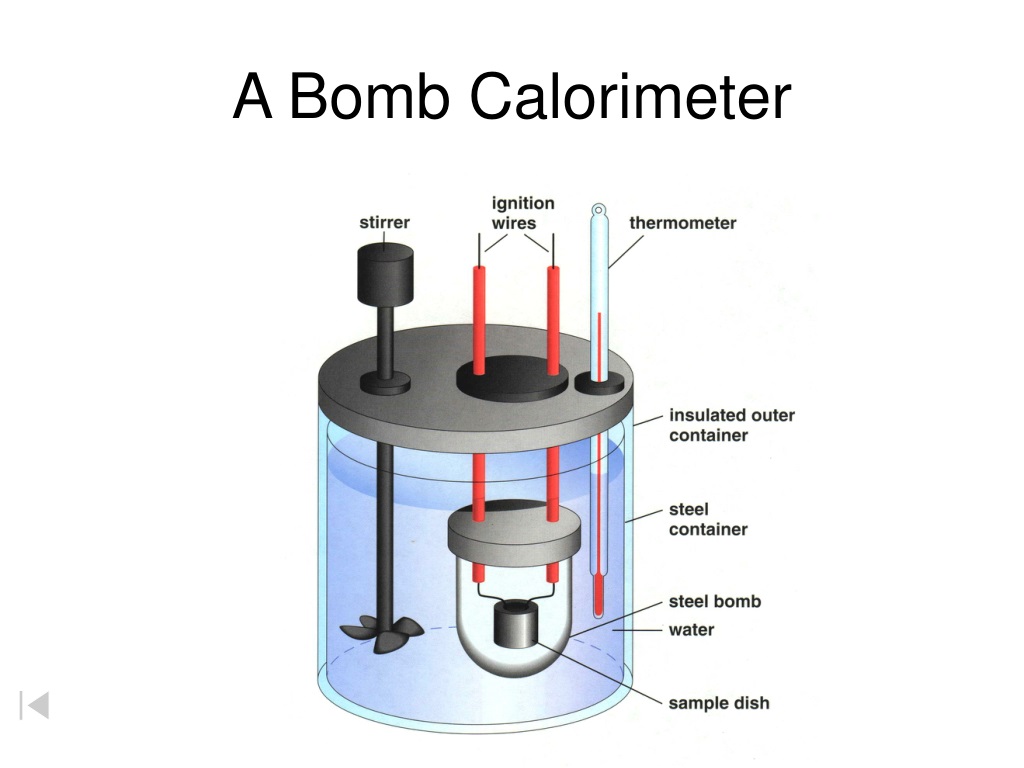
Bomb Calorimeter Range . When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. The consequence of the calculation is called the amount of combustion, calorification, or btu. Constant volume calorimetry, also know. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A tutorial guide on how to calculate the heat of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water.
from www.slideserve.com
This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. Constant volume calorimetry, also know. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. The consequence of the calculation is called the amount of combustion, calorification, or btu.
PPT Calorimetry PowerPoint Presentation, free download ID9276632
Bomb Calorimeter Range A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The consequence of the calculation is called the amount of combustion, calorification, or btu. This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification,. Bomb Calorimeter Range.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Range A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Constant volume calorimetry, also know. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. Describe a simple calorimeter and explain how. Bomb Calorimeter Range.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. This lab demonstrates one of the most common. The consequence of the calculation is called the. Bomb Calorimeter Range.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Range Constant volume calorimetry, also know. The consequence of the calculation is called the amount of combustion, calorification, or btu. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c.. Bomb Calorimeter Range.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. This lab demonstrates one of the most common. Constant volume calorimetry, also know. A tutorial guide. Bomb Calorimeter Range.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Range Constant volume calorimetry, also know. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification, or btu. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the. Bomb Calorimeter Range.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimeter Range Constant volume calorimetry, also know. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The consequence of the calculation is called. Bomb Calorimeter Range.
From dir.indiamart.com
Bomb Calorimeters at Best Price in India Bomb Calorimeter Range Constant volume calorimetry, also know. This lab demonstrates one of the most common. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A tutorial guide on how to calculate the heat of combustion. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the. Bomb Calorimeter Range.
From www.indiamart.com
Bomb Calorimeter at best price in New Delhi ID 15510167562 Bomb Calorimeter Range This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A tutorial guide on how to calculate the heat of combustion. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c.. Bomb Calorimeter Range.
From ddscalorimeters.com
DDS Calorimeters Products Range Oxygen Bomb Calorimeters Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. The consequence of the calculation is called the amount of combustion, calorification, or btu. This lab. Bomb Calorimeter Range.
From www.indiamart.com
Digital Bomb Calorimeter at best price in Mumbai by Sintrex Corporation Bomb Calorimeter Range Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. The consequence of the calculation is called the amount of combustion, calorification, or btu. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. This lab. Bomb Calorimeter Range.
From ddscalorimeters.com
DDS Calorimeters Products Range Oxygen Bomb Calorimeters Bomb Calorimeter Range This lab demonstrates one of the most common. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A bomb calorimeter is used to measure, under. Bomb Calorimeter Range.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an. Bomb Calorimeter Range.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Range Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. When 0.963 g of benzene, c 6 h 6, is burned. Bomb Calorimeter Range.
From people.chem.umass.edu
to Adobe GoLive 6 Bomb Calorimeter Range A tutorial guide on how to calculate the heat of combustion. The consequence of the calculation is called the amount of combustion, calorification, or btu. Constant volume calorimetry, also know. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. Describe a simple calorimeter and explain how it is employed. Bomb Calorimeter Range.
From www.indiamart.com
Automatic Advanced Bomb Calorimeter, For Laboratory, Model Name/Number Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it. Bomb Calorimeter Range.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Range A tutorial guide on how to calculate the heat of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter. Bomb Calorimeter Range.
From www.indiamart.com
AI Scientific Oxygen Bomb Calorimeter, Model AI3689, Rs 655000 /unit Bomb Calorimeter Range A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This lab demonstrates one of the most common. The consequence of the calculation is. Bomb Calorimeter Range.
From www.youtube.com
MUST WATCH!! DDS Oxygen Bomb Calorimeter Systems Range DDS Bomb Calorimeter Range A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The consequence of the calculation is called the amount of combustion, calorification, or btu. Constant volume calorimetry, also know. A tutorial guide on how to calculate the heat of combustion. When. Bomb Calorimeter Range.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter Range A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb. Bomb Calorimeter Range.
From dir.indiamart.com
Digital Bomb Calorimeter at Best Price in India Bomb Calorimeter Range This lab demonstrates one of the most common. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Constant volume calorimetry, also know. The consequence of the calculation is called the amount of combustion, calorification, or btu. When 0.963 g of. Bomb Calorimeter Range.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Bomb Calorimeter Range A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification, or btu. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by. Bomb Calorimeter Range.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Range This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere. Bomb Calorimeter Range.
From www.tradeindia.com
Automatic Bomb Calorimeter Test Range Accurate / Normal at Best Bomb Calorimeter Range This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification, or btu. A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. Constant volume calorimetry, also know. Describe a. Bomb Calorimeter Range.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter Range The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature. Bomb Calorimeter Range.
From www.indiamart.com
Digital Bomb Calorimeter Model CC01/M2, डिजिटल बम कैलोरीमीटर Bomb Calorimeter Range This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A tutorial guide on how to calculate the heat of combustion. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c.. Bomb Calorimeter Range.
From www.animalia-life.club
Calorimeter Diagram Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A tutorial guide on how to calculate the heat of combustion. Describe a simple calorimeter and. Bomb Calorimeter Range.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Range The consequence of the calculation is called the amount of combustion, calorification, or btu. This lab demonstrates one of the most common. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under. Bomb Calorimeter Range.
From ddscalorimeters.com
CAL3KF Calorimeter Bomb Calorimeter Range The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Constant volume calorimetry, also know. This lab demonstrates one of the most common. Bomb calorimeter was used. Bomb Calorimeter Range.
From ddscalorimeters.com
DDS Calorimeters Products Range Oxygen Bomb Calorimeters Bomb Calorimeter Range The consequence of the calculation is called the amount of combustion, calorification, or btu. A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. Describe a simple calorimeter and explain how it is employed. Bomb Calorimeter Range.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Range This lab demonstrates one of the most common. Bomb calorimeter was used to measure calorific value of fuel samples and experiment was performed as per astm d240 standard. Constant volume calorimetry, also know. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. The consequence of. Bomb Calorimeter Range.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Range A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A tutorial guide on how to calculate the heat of combustion. Constant volume calorimetry, also know. When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter,. Bomb Calorimeter Range.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Range When 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by. Bomb Calorimeter Range.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Range The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This lab demonstrates one of the most common. Constant volume calorimetry, also know. When 0.963 g of. Bomb Calorimeter Range.
From ddscalorimeters.com
DDS Calorimeters Products Range Oxygen Bomb Calorimeters Bomb Calorimeter Range A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This lab demonstrates one of the most common. The consequence of the calculation is called the amount of combustion, calorification, or btu. When 0.963 g of benzene, c 6 h 6,. Bomb Calorimeter Range.