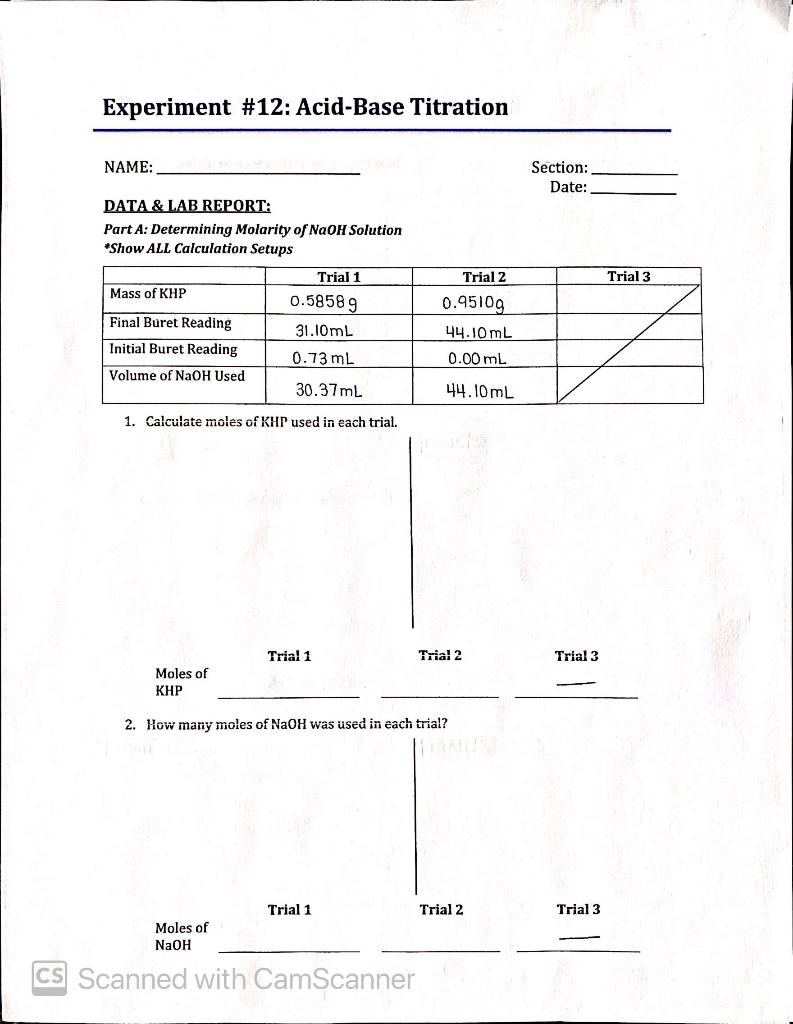
Acid Base Titration Gizmo Answers . A titration was performed using 1.00 m. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette,. To begin, check that 1 m naoh is. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is.
from moniquearesmeza.blogspot.com
To begin, check that 1 m naoh is selected for the burette,. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.
Acid Base Titration Lab Report Answers MoniquearesMeza
Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution.
From www.transtutors.com
(Solved) Student Exploration Titration of a Weak Acid bases Get the Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. To begin, check that 1 m naoh is selected for the burette,. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmotm, you will use. Acid Base Titration Gizmo Answers.
From www.studocu.com
Titration (Gizmo) Lab 201 Titration (Gizmo) Lab Vocabulary acid Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmo, you will use indicators to show how acids. Acid Base Titration Gizmo Answers.
From studyfinder.org
Acid Base Titration Pre Lab Answers Study Finder Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. A. Acid Base Titration Gizmo Answers.
From www.numerade.com
SOLVED Student Exploration Titration Vocabulary acid, analyte, base Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. A. Acid Base Titration Gizmo Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmotm, you will use indicators to show how acids. Acid Base Titration Gizmo Answers.
From www.chegg.com
Solved Activity C Weak acids and bases Get the Gizmo ready Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m. in the titration gizmo,. Acid Base Titration Gizmo Answers.
From worksheets.clipart-library.com
acid base titration worksheet answer key Worksheets Library Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is selected for the burette,. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm, you will. Acid Base Titration Gizmo Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titration Worksheet Answer Key Acid Base Titration Gizmo Answers A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. To begin, check that 1 m naoh is. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmo, you will use indicators to show how. Acid Base Titration Gizmo Answers.
From philo-khosravani.blogspot.com
Acid Base Titration Worksheet Answers philokhosravani Acid Base Titration Gizmo Answers in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of. Acid Base Titration Gizmo Answers.
From www.transtutors.com
(Solved) Student Exploration Titration Of A Weak Acid Bases Get The Acid Base Titration Gizmo Answers A titration was performed using 1.00 m. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. quiz yourself with questions. Acid Base Titration Gizmo Answers.
From www.transtutors.com
(Solved) Student Exploration Titration of a Weak Acid bases Get the Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. A. Acid Base Titration Gizmo Answers.
From education2research.com
The Secret to Mastering Titration Unlocking the Gizmo Answer Key Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.. Acid Base Titration Gizmo Answers.
From educacion.cc
Explore Learning Titration Gizmo Answer Key (2024). Online Education Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. To begin,. Acid Base Titration Gizmo Answers.
From dxodcsayx.blob.core.windows.net
Titration Lab Answers Gizmo at Janyce Lewis blog Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A process. Acid Base Titration Gizmo Answers.
From www.studocu.com
Gizmo Acids and Basesp HAnalysis SE Name Date Student Exploration Acid Base Titration Gizmo Answers A titration was performed using 1.00 m. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette,. A process. Acid Base Titration Gizmo Answers.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. To. Acid Base Titration Gizmo Answers.
From www.studocu.com
Titration SE Key Gizmo Answers 2019 Titration Answer Key Vocabulary Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. To begin, check that 1 m naoh is. in the titration gizmotm, you will use indicators to show how acids. Acid Base Titration Gizmo Answers.
From www.studocu.com
Titration gizmos Titration Vocabulary acid, analyte, base Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in. Acid Base Titration Gizmo Answers.
From tomdunnacademy.org
Acing the Titration Gizmo Quiz Unveiling the Perfect Answers Acid Base Titration Gizmo Answers A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m. in the titration gizmo, you will use indicators to show how. Acid Base Titration Gizmo Answers.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators. Acid Base Titration Gizmo Answers.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Gizmo Answers A titration was performed using 1.00 m. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm,. Acid Base Titration Gizmo Answers.
From studyfinder.org
Unveiling Acid Base Titration Pre Lab Answers A Comprehensive Guide Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.. Acid Base Titration Gizmo Answers.
From www.scribd.com
gizmo titration worksheet Titration Ph Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.. Acid Base Titration Gizmo Answers.
From www.scribd.com
Acid Base Titration PDF Ph Chemistry Acid Base Titration Gizmo Answers A titration was performed using 1.00 m. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. in the titration gizmotm, you will use indicators to show how acids. Acid Base Titration Gizmo Answers.
From www.numerade.com
SOLVED Student Exploration Titration Vocabulary acid, analyte, base Acid Base Titration Gizmo Answers A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. To begin, check that 1 m naoh is. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. quiz yourself with questions and answers for titration gizmo assessment,. Acid Base Titration Gizmo Answers.
From www.transtutors.com
(Solved) Student Exploration Titration Of A Weak Acid Bases Get The Acid Base Titration Gizmo Answers A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. . Acid Base Titration Gizmo Answers.
From studyfinder.org
Mastering Titrations Unlocking the Student Exploration Gizmo with Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. To begin, check that 1 m naoh is selected for the burette,. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A process in which a chemical reaction is used to measure the concentration or to determine the identity of a. Acid Base Titration Gizmo Answers.
From studyfinder.org
Mastering Acid Base Titration Your Comprehensive Worksheet Answer Key Acid Base Titration Gizmo Answers A process in which a chemical reaction is used to measure the concentration or to determine the identity of a solution. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. To begin, check that 1 m naoh is. To begin, check that 1 m naoh is. in the titration. Acid Base Titration Gizmo Answers.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Acid Base Titration Gizmo Answers in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for. Acid Base Titration Gizmo Answers.
From www.chegg.com
Solved Activity A Acids and bases Get the Gizmo ready • Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. A. Acid Base Titration Gizmo Answers.
From education2research.com
Understanding Acid Base Titration Pre Lab Questions and Answers Unraveled Acid Base Titration Gizmo Answers quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration. Acid Base Titration Gizmo Answers.
From studyfinder.org
The Ultimate Guide to Understanding and Interpreting Titration Curves Acid Base Titration Gizmo Answers A titration was performed using 1.00 m. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. in the titration gizmo, you will use indicators to show how acids are. Acid Base Titration Gizmo Answers.
From www.transtutors.com
(Solved) Student Exploration Titration Of A Weak Acid Bases Get The Acid Base Titration Gizmo Answers quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. A titration. Acid Base Titration Gizmo Answers.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Acid Base Titration Gizmo Answers To begin, check that 1 m naoh is. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. A titration was performed using 1.00 m. To begin, check that 1 m naoh is. in the titration gizmo, you will use indicators to show how acids are neutralized by bases, and. Acid Base Titration Gizmo Answers.
From dxodcsayx.blob.core.windows.net
Titration Lab Answers Gizmo at Janyce Lewis blog Acid Base Titration Gizmo Answers 0.13 m the reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 →. To begin, check that 1 m naoh is selected for the burette,. quiz yourself with questions and answers for titration gizmo assessment, so you can be ready for test day. To begin, check that 1 m naoh is. in. Acid Base Titration Gizmo Answers.