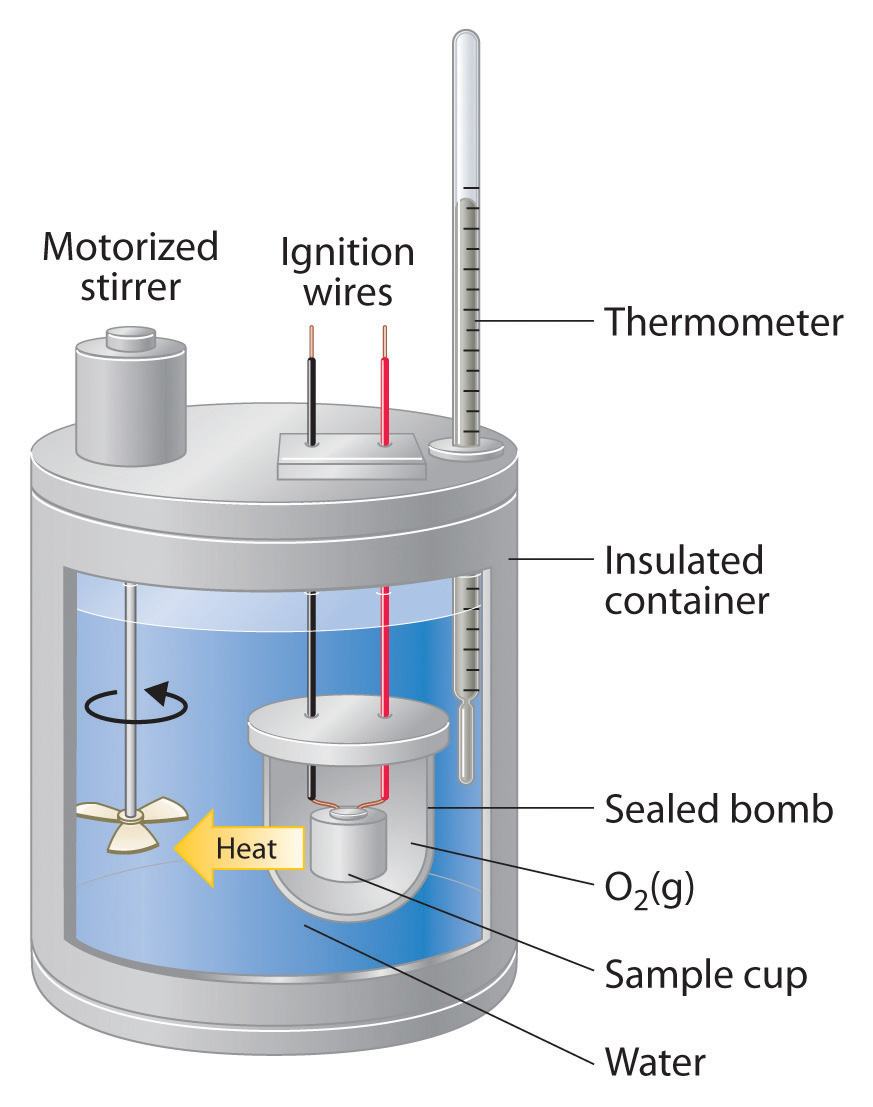
Calorimetry Tutorial . This chemistry video tutorial explains how to solve basic calorimetry problems. To do so, the heat is exchanged with a calibrated object (calorimeter). It discusses how to calculate the heat energy. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The surroundings are outside of. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. An adiabatic calorimeter 1 is. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). How to calculate enthalpy change using calorimetry method. Energy change, specific heat capacity, temperature of surroundings. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change measured by the calorimeter is used to derive the
from saylordotorg.github.io
It discusses how to calculate the heat energy. Energy change, specific heat capacity, temperature of surroundings. The temperature change measured by the calorimeter is used to derive the To do so, the heat is exchanged with a calibrated object (calorimeter). This chemistry video tutorial explains how to solve basic calorimetry problems. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). How to calculate enthalpy change using calorimetry method. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
Calorimetry
Calorimetry Tutorial This chemistry video tutorial explains how to solve basic calorimetry problems. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). An adiabatic calorimeter 1 is. It discusses how to calculate the heat energy. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). The surroundings are outside of. Energy change, specific heat capacity, temperature of surroundings. How to calculate enthalpy change using calorimetry method. This chemistry video tutorial explains how to solve basic calorimetry problems. The temperature change measured by the calorimeter is used to derive the
From www.slideserve.com
PPT 10.3.1 Specific Heat and Calorimetry PowerPoint Presentation Calorimetry Tutorial Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The surroundings are outside of. Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change measured by the calorimeter is used to derive the An adiabatic calorimeter 1 is. This tutorial covers how to. Calorimetry Tutorial.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Calorimetry Tutorial Calorimetry is used to measure amounts of heat transferred to or from a substance. It discusses how to calculate the heat energy. The temperature change measured by the calorimeter is used to derive the This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. A system is the specific place where a reaction or process is occurring. Calorimetry Tutorial.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Tutorial This chemistry video tutorial explains how to solve basic calorimetry problems. An adiabatic calorimeter 1 is. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). How to calculate enthalpy change using calorimetry method. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. Calorimetry is a field of thermochemistry that. Calorimetry Tutorial.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Tutorial The surroundings are outside of. It discusses how to calculate the heat energy. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. Calorimetry is used to measure amounts of heat transferred to or from a substance. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). The temperature. Calorimetry Tutorial.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Tutorial Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using calorimetry method. To do so, the heat is exchanged with a calibrated object (calorimeter). An adiabatic calorimeter 1 is. The temperature change measured by the calorimeter is used to derive the It discusses how to. Calorimetry Tutorial.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Tutorial The surroundings are outside of. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It discusses how to calculate the heat energy. Calorimetry is used to measure amounts of heat transferred to or from a substance. Energy change, specific heat capacity, temperature of surroundings. This tutorial covers how to calculate. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Tutorial Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using calorimetry method. To do so, the heat is exchanged with a calibrated object (calorimeter). Energy change, specific heat capacity, temperature of. Calorimetry Tutorial.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Tutorial Calorimetry is used to measure amounts of heat transferred to or from a substance. How to calculate enthalpy change using calorimetry method. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. An adiabatic calorimeter 1 is. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). To do so, the. Calorimetry Tutorial.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry Tutorial An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). A system is the specific place where a reaction or process is occurring (eg inside calorimeter). An adiabatic calorimeter 1 is. Energy change, specific heat capacity, temperature of surroundings. It discusses how to calculate the heat energy. This tutorial covers how to. Calorimetry Tutorial.
From studylib.net
Calorimetry Calorimetry Tutorial It discusses how to calculate the heat energy. The temperature change measured by the calorimeter is used to derive the An adiabatic calorimeter 1 is. Energy change, specific heat capacity, temperature of surroundings. This chemistry video tutorial explains how to solve basic calorimetry problems. How to calculate enthalpy change using calorimetry method. This tutorial covers how to calculate enthalpies of. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry C ontents Basic Concept Example Whiteboards Bomb Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. It discusses how to calculate the heat energy. This chemistry video tutorial explains how to solve basic calorimetry problems. The temperature change. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Tutorial The temperature change measured by the calorimeter is used to derive the The surroundings are outside of. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Tutorial An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. This chemistry video tutorial explains how to solve basic. Calorimetry Tutorial.
From studylib.net
Calorimetry Tutorial Calorimetry Tutorial Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change measured by the calorimeter is used to derive the This chemistry video tutorial explains how to solve basic calorimetry problems. To do so, the heat is exchanged with a calibrated object (calorimeter). An calorimeter is a device used to measure the enthalpy change. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Tutorial An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. The surroundings are outside of. To do so, the heat is exchanged with a calibrated object (calorimeter). How to calculate enthalpy change using calorimetry method. Energy change, specific heat. Calorimetry Tutorial.
From www.open.edu
OLCreate OpenSTEM Africa Ghana Background (Calorimetry combustion Calorimetry Tutorial An adiabatic calorimeter 1 is. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). The surroundings are outside of. To do so, the heat is exchanged with a calibrated object (calorimeter). This. Calorimetry Tutorial.
From www.hanlin.com
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Tutorial Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). Energy change, specific heat capacity, temperature of surroundings. To do so, the heat is exchanged with a calibrated object (calorimeter). This chemistry video. Calorimetry Tutorial.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Tutorial An adiabatic calorimeter 1 is. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. This chemistry video tutorial explains how to solve basic calorimetry problems. The surroundings are outside of. It discusses how to. Calorimetry Tutorial.
From users.highland.edu
Calorimetry Calorimetry Tutorial An adiabatic calorimeter 1 is. How to calculate enthalpy change using calorimetry method. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. The surroundings are outside of. Energy change, specific heat capacity, temperature of surroundings. An calorimeter is a device used to measure. Calorimetry Tutorial.
From www.onenewspage.com
Calorimetry, Constant Volume, Bomb Calorimeter, One News Page VIDEO Calorimetry Tutorial The temperature change measured by the calorimeter is used to derive the It discusses how to calculate the heat energy. Energy change, specific heat capacity, temperature of surroundings. An adiabatic calorimeter 1 is. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). An. Calorimetry Tutorial.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. An adiabatic calorimeter 1 is. It discusses how to calculate the heat energy. The surroundings are outside of. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. This chemistry video tutorial explains how to solve. Calorimetry Tutorial.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Tutorial The surroundings are outside of. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is used to measure amounts of heat transferred to or from a substance. This chemistry video tutorial explains how to solve basic calorimetry problems. It discusses how to calculate the heat energy. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. An. Calorimetry Tutorial.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. Energy change, specific heat capacity, temperature of surroundings. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). A system is the specific. Calorimetry Tutorial.
From www.youtube.com
09.05 Calorimetry YouTube Calorimetry Tutorial Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific heat capacity, temperature of surroundings. The temperature change measured by the calorimeter is used to derive the An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). To do so,. Calorimetry Tutorial.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Tutorial Energy change, specific heat capacity, temperature of surroundings. Calorimetry is used to measure amounts of heat transferred to or from a substance. It discusses how to calculate the heat energy. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature change measured by the calorimeter is used to derive the An calorimeter is a device used. Calorimetry Tutorial.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). The temperature change measured by the calorimeter is used to derive the Energy change, specific heat capacity, temperature of surroundings. It discusses how to calculate the heat energy. To do so, the heat is exchanged with. Calorimetry Tutorial.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. The surroundings are outside of. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimetry Tutorial.
From saylordotorg.github.io
Calorimetry Calorimetry Tutorial Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. An adiabatic calorimeter 1 is. Calorimetry is used to measure amounts of heat transferred to or from a substance. The surroundings are outside of. The temperature change measured by the calorimeter is used to derive the This chemistry video tutorial explains. Calorimetry Tutorial.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Tutorial How to calculate enthalpy change using calorimetry method. An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). An adiabatic calorimeter 1 is. It discusses how to calculate the heat energy. To do so, the heat is exchanged with a calibrated object (calorimeter). A system is the specific place where a reaction. Calorimetry Tutorial.
From www.youtube.com
Calorimetry YouTube Calorimetry Tutorial Energy change, specific heat capacity, temperature of surroundings. This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using calorimetry method. Calorimetry is used to measure amounts of heat transferred to or from. Calorimetry Tutorial.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Tutorial The surroundings are outside of. The temperature change measured by the calorimeter is used to derive the Calorimetry is used to measure amounts of heat transferred to or from a substance. This chemistry video tutorial explains how to solve basic calorimetry problems. An adiabatic calorimeter 1 is. Calorimetry is a field of thermochemistry that measures the amount of heat involved. Calorimetry Tutorial.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Tutorial An adiabatic calorimeter 1 is. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. This chemistry video tutorial explains how to. Calorimetry Tutorial.
From www.studocu.com
Chem 113 Tutorial 3 Calorimetry Chem 113 Tutorial 3 Calorimetry Track Calorimetry Tutorial The temperature change measured by the calorimeter is used to derive the How to calculate enthalpy change using calorimetry method. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). An calorimeter is a device used to measure the enthalpy change of a chemical reaction (heat of reaction). Energy change, specific heat capacity, temperature. Calorimetry Tutorial.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Tutorial This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes. This chemistry video tutorial explains how to solve basic calorimetry problems. The temperature change measured by the calorimeter is used to derive the Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific heat capacity,. Calorimetry Tutorial.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Tutorial Calorimetry is used to measure amounts of heat transferred to or from a substance. Energy change, specific heat capacity, temperature of surroundings. How to calculate enthalpy change using calorimetry method. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the heat is exchanged with a calibrated object. Calorimetry Tutorial.