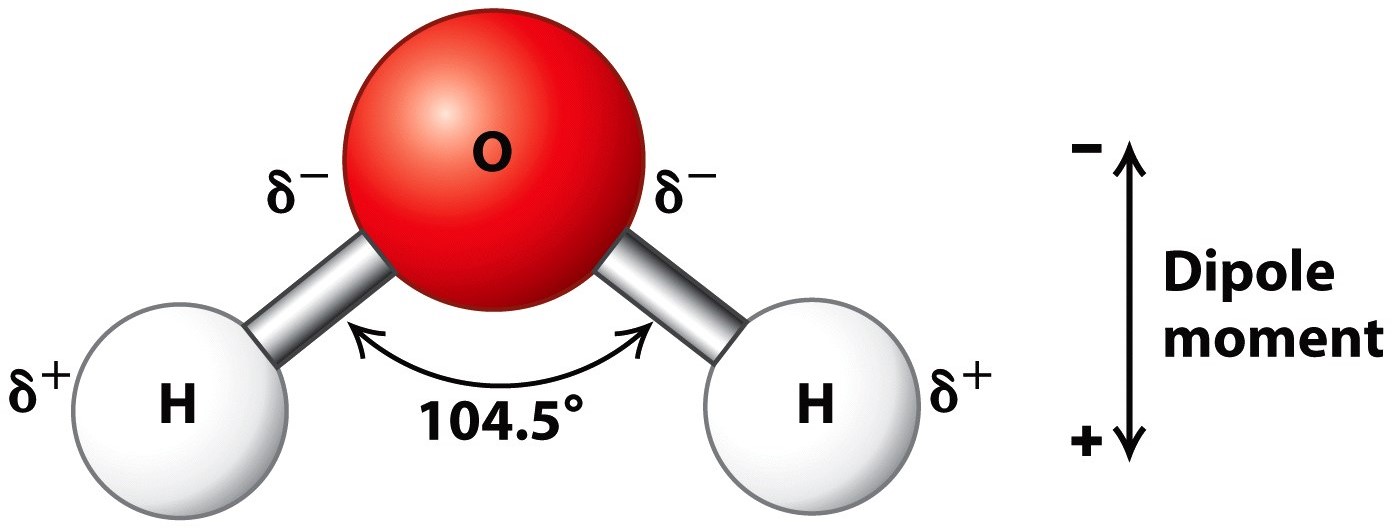
What Shape Is A Water Molecule . Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The bent shape of the water molecule is critical because the polar Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule, visualized three different ways: Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. This is due to the fact that there are two pairs of shared electrons. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
from www.linkedin.com
Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The bent shape of the water molecule is critical because the polar This is due to the fact that there are two pairs of shared electrons. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. The water molecule, visualized three different ways: Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons.
02.2 Properties of Water
What Shape Is A Water Molecule This is due to the fact that there are two pairs of shared electrons. This is due to the fact that there are two pairs of shared electrons. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. The bent shape of the water molecule is critical because the polar Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The water molecule, visualized three different ways:
From www.scienceabc.com
Is Water Polar Or Nonpolar? » ScienceABC What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The bent shape of the water molecule is critical because the polar Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how to draw the lewis structure of h2o with two. What Shape Is A Water Molecule.
From digitalpaxton.org
water molecule What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The water molecule, visualized three different ways: Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. This is. What Shape Is A Water Molecule.
From pixels.com
H2O Water molecule model and chemical formula Digital Art by Peter What Shape Is A Water Molecule Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule, visualized three different ways: Learn how water is a polar molecule with a bent shape and two lone pairs. What Shape Is A Water Molecule.
From aviewfromtheright.com
Water, Water Everywhere… Science, POLITICS, & Religion What Shape Is A Water Molecule Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. This is due to the fact that there are two pairs of shared electrons. The water molecule, visualized three. What Shape Is A Water Molecule.
From logyofbio.blogspot.com
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule What Shape Is A Water Molecule This is due to the fact that there are two pairs of shared electrons. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. The bent shape of the water molecule is critical. What Shape Is A Water Molecule.
From www.vectorstock.com
H2o water molecule Royalty Free Vector Image VectorStock What Shape Is A Water Molecule This is due to the fact that there are two pairs of shared electrons. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule, visualized three different ways: Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The bent. What Shape Is A Water Molecule.
From socratic.org
Question edb9f Socratic What Shape Is A Water Molecule Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. This is due to the fact that there are two pairs of shared electrons. Learn how these factors affect. What Shape Is A Water Molecule.
From lah.elearningontario.ca
SBI4U What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The bent shape of the water molecule is critical because the polar Learn how to draw the lewis structure of h2o. What Shape Is A Water Molecule.
From www.linkedin.com
02.2 Properties of Water What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar This is due to the fact that there are two pairs of shared electrons. The water molecule, visualized three different ways: Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how to draw the lewis structure of h2o. What Shape Is A Water Molecule.
From www.britannica.com
Water Definition, Chemical Formula, Structure, Molecule, & Facts What Shape Is A Water Molecule Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The water molecule, visualized three different ways: Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Water is a compound of hydrogen and oxygen that exists in three. What Shape Is A Water Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure What Shape Is A Water Molecule Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. This is due to the fact that there are two pairs of shared electrons. Water is a compound of hydrogen and. What Shape Is A Water Molecule.
From oxbridgeapplications.com
A Chemistry Puzzle Oxbridge Applications What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The bent shape of the water molecule is critical because the polar Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Water is a compound of hydrogen and oxygen that. What Shape Is A Water Molecule.
From www.scienceabc.com
Is Water Polar Or Nonpolar? » ScienceABC What Shape Is A Water Molecule The water molecule, visualized three different ways: Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how to draw the lewis structure of h2o with two single bonds and two lone. What Shape Is A Water Molecule.
From www.alamy.com
Water Molecule Stock Photos & Water Molecule Stock Images Alamy What Shape Is A Water Molecule Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule,. What Shape Is A Water Molecule.
From www.dreamstime.com
H2O, Water Molecule Illustration Stock Vector Illustration of core What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. This is due to the fact that there are two pairs of shared electrons. The bent shape of the water. What Shape Is A Water Molecule.
From kgrowth.blogspot.com
KGrowth What can we know about water molecule? What Shape Is A Water Molecule The water molecule, visualized three different ways: Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. This is due to the fact that there are two pairs of shared electrons. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the. What Shape Is A Water Molecule.
From www.turbosquid.com
Water molecule 3D TurboSquid 1164398 What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar The water molecule, visualized three different ways: Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.. What Shape Is A Water Molecule.
From pixels.com
Water Molecule Digital Art by Laguna Design Pixels What Shape Is A Water Molecule Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The bent shape of the water molecule is critical because the polar Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how to draw the lewis structure of h2o with two. What Shape Is A Water Molecule.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated What Shape Is A Water Molecule Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The. What Shape Is A Water Molecule.
From zulfafiqah95.blogspot.com.es
Assalamualaikum.... What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The bent shape of the water molecule is critical because the polar This is due to the fact that there are two pairs of shared electrons. Learn how to draw the lewis structure of h2o with two single bonds and two. What Shape Is A Water Molecule.
From www.alamy.com
Water molecule Stock Photo Alamy What Shape Is A Water Molecule Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. The bent shape of the water molecule is critical because the polar The water molecule, visualized three different ways: This is due to the fact that there are two pairs of shared electrons. Learn how these factors affect the polarity and. What Shape Is A Water Molecule.
From beyondpenguins.ehe.osu.edu
Teaching about Snowflakes A Flurry of Ideas for Science and Math What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. This is due to the fact that there. What Shape Is A Water Molecule.
From www.medicalsciencenavigator.com
Physiology of Cell Signaling What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. The bent shape of the water molecule is critical because the polar Water is a polar molecule because of its. What Shape Is A Water Molecule.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. What Shape Is A Water Molecule Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The water molecule, visualized three different ways: This is due to the fact that there are two pairs of shared electrons. Learn. What Shape Is A Water Molecule.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Learn how to draw the lewis structure of. What Shape Is A Water Molecule.
From www.sciencephoto.com
Water molecule Stock Image A700/0381 Science Photo Library What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar The water molecule, visualized three different ways: Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. Learn how to draw. What Shape Is A Water Molecule.
From www.dreamstime.com
Water Molecule. Molecule Structure. Atomic H2O. Stock Vector What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. The. What Shape Is A Water Molecule.
From www.researchgate.net
Surface tension and capillary forces acting on papermaking fibres What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The bent shape of the water molecule is critical because the polar Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. This is due to the fact that there. What Shape Is A Water Molecule.
From chemistrytalk.org
Physical & Chemical Properties of Water ChemTalk What Shape Is A Water Molecule Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The bent shape of the water molecule is critical because the polar The water molecule, visualized three different ways: Learn how to draw the lewis structure of h2o with two single bonds and two lone pairs of electrons. Learn how. What Shape Is A Water Molecule.
From www.quirkyscience.com
Bent Water Molecule Why Is It Bent? What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The water molecule, visualized three different ways: Learn how to draw the lewis structure of h2o with two single bonds and. What Shape Is A Water Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure What Shape Is A Water Molecule The bent shape of the water molecule is critical because the polar This is due to the fact that there are two pairs of shared electrons. Water is a compound of hydrogen and oxygen that exists in three states and has many unique properties. The water molecule, visualized three different ways: Learn how to draw the lewis structure of h2o. What Shape Is A Water Molecule.
From jeopardylabs.com
Molecular Jeopardy Template What Shape Is A Water Molecule Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. The bent shape of the water molecule is critical because the polar Water is a polar molecule because of its. What Shape Is A Water Molecule.
From sketchfab.com
H2O Molecule Download Free 3D model by Mehdi Mirzaie (MehdiMM What Shape Is A Water Molecule Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule, visualized three different ways: Water is a compound of hydrogen and oxygen that exists in three states. What Shape Is A Water Molecule.
From pixels.com
Water Molecule Structure Photograph by Inna Bigun/science Photo Library What Shape Is A Water Molecule Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. Learn how these factors affect the polarity and solubility of water and see examples of polar and nonpolar molecules. The water molecule, visualized three different ways: Water is a compound of hydrogen and oxygen that exists in three states and. What Shape Is A Water Molecule.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated What Shape Is A Water Molecule Learn how water is a polar molecule with a bent shape and two lone pairs of electrons on the oxygen atom. This is due to the fact that there are two pairs of shared electrons. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The water molecule, visualized three. What Shape Is A Water Molecule.