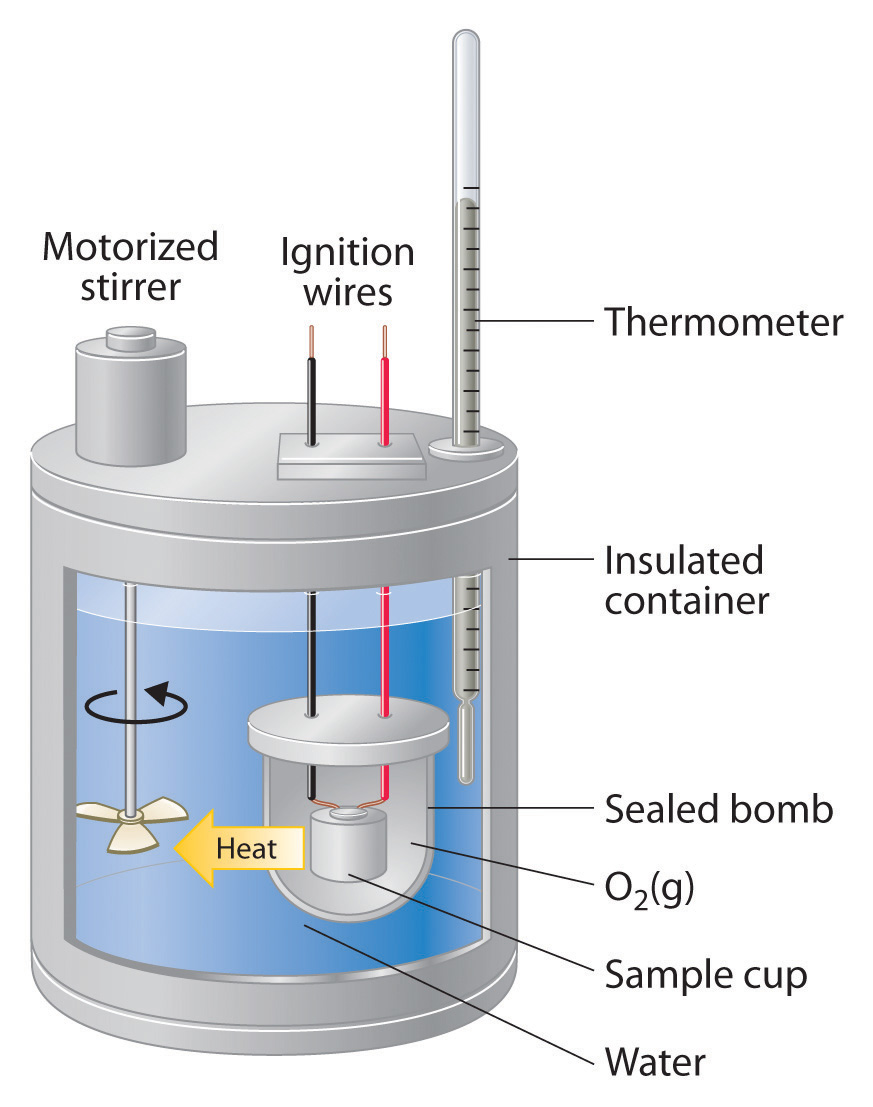
Calorimetry Chemical Engineering . In the calorimeter, the enthalpy. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. For example, when an exothermic reaction occurs in solution in a. Applications of calorimetry to different. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Fundamentals, instrumentation and applications, first edition. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from sites.google.com
Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. In the calorimeter, the enthalpy. For example, when an exothermic reaction occurs in solution in a. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Fundamentals, instrumentation and applications, first edition. Applications of calorimetry to different. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds.
Calorimetry Preliminary HSC Chemistry
Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. For example, when an exothermic reaction occurs in solution in a. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In the calorimeter, the enthalpy. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Fundamentals, instrumentation and applications, first edition. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Applications of calorimetry to different.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Chemical Engineering Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. In the calorimeter, the enthalpy. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Simply put, reaction calorimetry is a. Calorimetry Chemical Engineering.
From pubs.rsc.org
Thermal characterization of highly exothermic flash chemistry in a continuous flow calorimeter Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Fundamentals, instrumentation and applications, first edition. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Reaction calorimetry can be. Calorimetry Chemical Engineering.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Chemical Engineering Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an. Calorimetry Chemical Engineering.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Chemical Engineering In the calorimeter, the enthalpy. For example, when an exothermic reaction occurs in solution in a. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Fundamentals, instrumentation and applications, first edition. A calorimeter is. Calorimetry Chemical Engineering.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Chemical Engineering In the calorimeter, the enthalpy. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Simply put, reaction. Calorimetry Chemical Engineering.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Chemistry Calorimetry Chemical Engineering Applications of calorimetry to different. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Fundamentals, instrumentation and applications, first edition. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. A calorimeter is a device used to measure the amount. Calorimetry Chemical Engineering.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Chemical Engineering Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Fundamentals, instrumentation and applications, first edition. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Reaction calorimetry can be employed for kinetic studies of complex. Calorimetry Chemical Engineering.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. For example, when an exothermic reaction occurs in solution in a. Fundamentals, instrumentation and applications, first edition. Simply put, reaction calorimetry. Calorimetry Chemical Engineering.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application Calorimetry Chemical Engineering Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Applications of calorimetry to different. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a thermodynamic approach that directly measures the heat released or. Calorimetry Chemical Engineering.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Chemical Engineering Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Fundamentals, instrumentation and applications, first edition. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Simply put, reaction calorimetry is a. Calorimetry Chemical Engineering.
From www.youtube.com
CALORIMETRY ENGINEERING THERMODYNAMICS SOLVED PROBLEM ENGINEERING PHYSICAL CHEMISTRY Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Reaction calorimetry can be employed. Calorimetry Chemical Engineering.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Calorimetry Chemical Engineering Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Fundamentals, instrumentation and applications, first edition. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry. Calorimetry Chemical Engineering.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Chemical Engineering In the calorimeter, the enthalpy. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Fundamentals, instrumentation and applications, first edition. Calorimetry. Calorimetry Chemical Engineering.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimetry Chemical Engineering Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Applications of calorimetry to. Calorimetry Chemical Engineering.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Chemical Engineering Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of. Calorimetry Chemical Engineering.
From printablecampusreises.z21.web.core.windows.net
How To Solve Calorimetry Problems Chemistry Calorimetry Chemical Engineering Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic reaction occurs in solution in a. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Applications of calorimetry to different.. Calorimetry Chemical Engineering.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimetry Chemical Engineering Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Reaction. Calorimetry Chemical Engineering.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Chemical Engineering Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Fundamentals, instrumentation and applications, first edition. For example, when an exothermic reaction occurs in solution in a. Simply put, reaction calorimetry is a discipline that enables chemists to. Calorimetry Chemical Engineering.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Applications of calorimetry to different. In the calorimeter, the enthalpy. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Calorimetry is a branch of science concerned. Calorimetry Chemical Engineering.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimetry Chemical Engineering Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. Calorimetry Chemical Engineering.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Applications of calorimetry to different. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry. Calorimetry Chemical Engineering.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Chemical Engineering Applications of calorimetry to different. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Fundamentals, instrumentation and applications, first edition. For example, when an exothermic. Calorimetry Chemical Engineering.
From users.highland.edu
Calorimetry Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Fundamentals, instrumentation and applications, first edition. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Calorimetry Chemical Engineering.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Chemical Engineering Fundamentals, instrumentation and applications, first edition. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. A calorimeter is a device used to measure the amount. Calorimetry Chemical Engineering.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Chemical Engineering Applications of calorimetry to different. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. In the calorimeter, the enthalpy. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Simply put, reaction calorimetry is a. Calorimetry Chemical Engineering.
From www.scribd.com
Gas Calorimeter Power Point PDF Branches Of Thermodynamics Chemical Engineering Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. In the calorimeter, the enthalpy. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Applications of calorimetry to different. Fundamentals, instrumentation and applications, first edition. To. Calorimetry Chemical Engineering.
From microinnova.com
Continuous Flow Reaction Calorimetry A Novel Tool For Industrial Flow Chemistry Microinnova Calorimetry Chemical Engineering For example, when an exothermic reaction occurs in solution in a. Applications of calorimetry to different. Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. To determine the enthalpy, stability, heat capacity, and. Calorimetry Chemical Engineering.
From www.sealengineering.no
Differential scanning calorimeter Seal Engineering Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Applications of calorimetry to different.. Calorimetry Chemical Engineering.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. For example, when an exothermic reaction occurs in solution in a. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing. Calorimetry Chemical Engineering.
From exovxxbwq.blob.core.windows.net
Calorimeter In Chemical Engineering at Elizabeth Hodgson blog Calorimetry Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Fundamentals, instrumentation and applications,. Calorimetry Chemical Engineering.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimetry Chemical Engineering Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Fundamentals, instrumentation and applications, first edition. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a thermodynamic approach that directly measures the heat. Calorimetry Chemical Engineering.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical Calorimetry Chemical Engineering Simply put, reaction calorimetry is a discipline that enables chemists to perform thermal and kinetic analysis of their desired process in small scales (e.g. For example, when an exothermic reaction occurs in solution in a. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Fundamentals, instrumentation and applications, first edition. Applications of. Calorimetry Chemical Engineering.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Chemical Engineering Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. Fundamentals, instrumentation and applications, first edition. In the calorimeter, the enthalpy. Applications of calorimetry to different. Allowing for simulation of failure scenarios safely), and. Calorimetry Chemical Engineering.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Calorimetry Chemical Engineering Fundamentals, instrumentation and applications, first edition. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Allowing for simulation of failure scenarios safely), and under realistic conditions (stirring, temperature, pressure, dosing ramps, etc.). Reaction calorimetry can be employed for kinetic studies of complex catalytic reactions. A calorimeter is a device used to measure the amount of heat involved. Calorimetry Chemical Engineering.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Chemical Engineering Applications of calorimetry to different. Calorimetry is a thermodynamic approach that directly measures the heat released or absorbed during formation and break of chemical bonds. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. For example, when an exothermic reaction occurs in solution in a. Reaction calorimetry can be employed for kinetic studies of. Calorimetry Chemical Engineering.