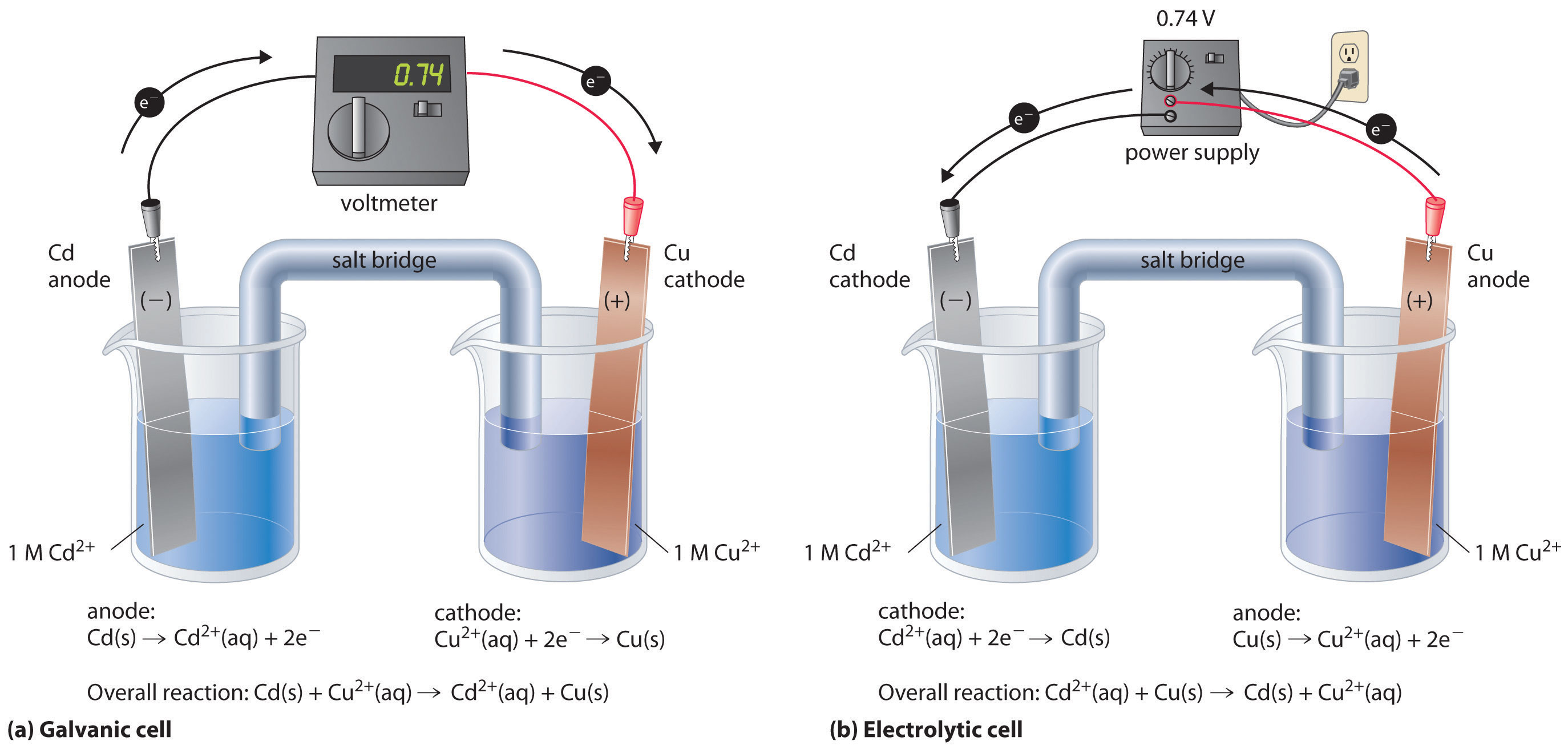
Define Electrode Electrolyte . Positively charged ions are called cations. Electrode and electrolyte are two essential components in electrochemical systems. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrode refers to a conductor through which electric. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrolytes are minerals that carry an electric charge. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Weak electrolytes partially ionize in water. An electrolyte is a substance that dissociates in water into charged particles called ions. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. They’re found in your blood, urine and sweat and are vital to specific processes that.
from chem.libretexts.org
Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes are minerals that carry an electric charge. Weak electrolytes partially ionize in water. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. An electrolyte is a substance that dissociates in water into charged particles called ions. They’re found in your blood, urine and sweat and are vital to specific processes that. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which electric.
Chapter 19.7 Electrolysis Chemistry LibreTexts
Define Electrode Electrolyte Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Positively charged ions are called cations. Weak electrolytes partially ionize in water. They’re found in your blood, urine and sweat and are vital to specific processes that. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which electric. An electrolyte is a substance that dissociates in water into charged particles called ions. Electrolytes are minerals that carry an electric charge. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Define Electrode Electrolyte Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. An electrolyte is a substance that dissociates in water into charged particles called ions. They’re found in your blood, urine and sweat. Define Electrode Electrolyte.
From www.pinterest.com
Electrolyte Easy Science Electrolytes, Easy science, Flashcards Define Electrode Electrolyte Electrolytes are minerals that carry an electric charge. Electrode and electrolyte are two essential components in electrochemical systems. They’re found in your blood, urine and sweat and are vital to specific processes that. Positively charged ions are called cations. Electrode refers to a conductor through which electric. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that. Define Electrode Electrolyte.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12, Electro Chemistry Define Electrode Electrolyte Positively charged ions are called cations. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. They’re found in your blood, urine and sweat and are vital to specific processes that. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in. Define Electrode Electrolyte.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Define Electrode Electrolyte Electrolytes are minerals that carry an electric charge. Electrode refers to a conductor through which electric. Weak electrolytes partially ionize in water. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions.. Define Electrode Electrolyte.
From ppt-online.org
Electrolysis презентация онлайн Define Electrode Electrolyte Electrode refers to a conductor through which electric. Electrode and electrolyte are two essential components in electrochemical systems. Weak electrolytes partially ionize in water. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. To start the great journey of electrolytes, we travel back into the history, and learn how the. Define Electrode Electrolyte.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry. YouTube Define Electrode Electrolyte Electrode refers to a conductor through which electric. An electrolyte is a substance that dissociates in water into charged particles called ions. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions.. Define Electrode Electrolyte.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Define Electrode Electrolyte To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode refers to a conductor through which electric. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes are. Define Electrode Electrolyte.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Define Electrode Electrolyte Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. They’re found. Define Electrode Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Positively charged ions are called cations. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. They’re found. Define Electrode Electrolyte.
From www.researchgate.net
Schematic of the (negative) electrodeelectrolyte interface in EDLCs... Download Scientific Define Electrode Electrolyte They’re found in your blood, urine and sweat and are vital to specific processes that. Electrolytes are minerals that carry an electric charge. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a. Define Electrode Electrolyte.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Electrode and electrolyte are two essential components in electrochemical systems. Positively charged ions are called cations. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. They’re found in your blood, urine and sweat and. Define Electrode Electrolyte.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition, Properties, Reactions Define Electrode Electrolyte Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Positively charged ions are called cations. Electrolyte, substance that conducts electric current as a result of dissociation into positively and. Define Electrode Electrolyte.
From www.youtube.com
6. Electrode and electrolyte defined (HSC chemistry) YouTube Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. They’re found in your blood, urine and sweat and are vital to specific processes that. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance. Define Electrode Electrolyte.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Define Electrode Electrolyte Electrode refers to a conductor through which electric. Positively charged ions are called cations. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. An electrolyte is a substance that. Define Electrode Electrolyte.
From stoplearn.com
Electrolysis Notes 2023 Define Electrode Electrolyte Positively charged ions are called cations. Electrode refers to a conductor through which electric. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Weak electrolytes partially ionize in water.. Define Electrode Electrolyte.
From wireenginepaul.z19.web.core.windows.net
Circuit Diagram Electrolysis Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which electric. They’re found in your blood, urine and sweat and are vital to specific processes that. Electrolytes are minerals that carry an electric charge. Positively charged ions are called. Define Electrode Electrolyte.
From www.thoughtco.com
How to Define Anode and Cathode Define Electrode Electrolyte Electrode and electrolyte are two essential components in electrochemical systems. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. They’re found in your blood, urine and sweat and are vital to specific processes that. To start the great journey of electrolytes, we travel back into the history, and learn how. Define Electrode Electrolyte.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Define Electrode Electrolyte Weak electrolytes partially ionize in water. Electrode refers to a conductor through which electric. Positively charged ions are called cations. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolytes are minerals that carry an electric charge. An electrolyte is a substance that dissociates in water into charged particles called. Define Electrode Electrolyte.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Positively charged ions are called cations. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. They’re found. Define Electrode Electrolyte.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Scientific Diagram Define Electrode Electrolyte Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which electric. They’re found. Define Electrode Electrolyte.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Define Electrode Electrolyte They’re found in your blood, urine and sweat and are vital to specific processes that. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes are minerals that carry an electric charge. An electrolyte is a substance that dissociates in water into charged particles called ions. Electrodes are conductive materials. Define Electrode Electrolyte.
From grammarbeast.com
Electrolyte vs Electrode Differences And Uses For Each One Define Electrode Electrolyte Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. An electrolyte is a substance that dissociates in water into charged particles called ions. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Positively charged ions are called cations.. Define Electrode Electrolyte.
From www.researchgate.net
Electrodeelectrolyte interface and charge injection. (A) Schematic... Download Scientific Diagram Define Electrode Electrolyte Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Weak electrolytes partially ionize in water. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrolytes are minerals that carry an. Define Electrode Electrolyte.
From quizunbestowed.z21.web.core.windows.net
Why Are Inert Electrodes Used In Electrolysis Define Electrode Electrolyte To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. An electrolyte is a substance that dissociates in water into charged particles called ions. Electrolytes are minerals that carry an electric charge. Weak electrolytes partially ionize in water. Electrode refers to a conductor through which electric. They’re found in. Define Electrode Electrolyte.
From exowfmfux.blob.core.windows.net
Standard Electrode Examples at Lucille Fitzsimmons blog Define Electrode Electrolyte Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Positively charged ions are called cations. Electrolytes are minerals that carry an electric charge. Electrode and electrolyte are two essential components in. Define Electrode Electrolyte.
From slideplayer.com
C3.4 Electrolysis and cells ppt download Define Electrode Electrolyte Electrolytes are minerals that carry an electric charge. They’re found in your blood, urine and sweat and are vital to specific processes that. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which electric. Pretty much any dissociation into ions between 0% and 100%. Define Electrode Electrolyte.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical... Download Scientific Diagram Define Electrode Electrolyte Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. An electrolyte is a substance that dissociates in water into charged particles called ions. They’re found in your blood, urine and. Define Electrode Electrolyte.
From guidepartexplored.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Define Electrode Electrolyte Electrode and electrolyte are two essential components in electrochemical systems. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. An electrolyte is a substance that dissociates in water into charged particles called ions. Electrolytes are minerals that carry an electric charge. To start the great journey of electrolytes, we travel. Define Electrode Electrolyte.
From www.shutterstock.com
Electrode Electrolyte Half Cell Chemistry Lesson เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 1459333040 Define Electrode Electrolyte Electrode refers to a conductor through which electric. Weak electrolytes partially ionize in water. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Electrode and electrolyte are two essential components in electrochemical systems. Positively charged ions are called cations. Electrolyte, substance that conducts electric current as a result of dissociation. Define Electrode Electrolyte.
From www.youtube.com
Electrochemistry 6 Type of Electrodes YouTube Define Electrode Electrolyte Weak electrolytes partially ionize in water. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode and electrolyte are two essential components in electrochemical systems. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Positively charged ions are. Define Electrode Electrolyte.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Define Electrode Electrolyte Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. They’re found in your blood, urine and sweat and are vital to specific processes that. Electrode and electrolyte are two essential components. Define Electrode Electrolyte.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Define Electrode Electrolyte An electrolyte is a substance that dissociates in water into charged particles called ions. Weak electrolytes partially ionize in water. Positively charged ions are called cations. They’re found in your blood, urine and sweat and are vital to specific processes that. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,.. Define Electrode Electrolyte.
From www.pinterest.com
What is Electrolysis and Electrolyte with examples. Teaching chemistry, Chemistry lessons Define Electrode Electrolyte Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which electric. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Positively charged ions are called cations. Electrodes are conductive materials allowing electric current flow,. Define Electrode Electrolyte.
From exouigbkb.blob.core.windows.net
Electrode Definition Simple at Lenard Gillis blog Define Electrode Electrolyte Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which electric. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. They’re found in your blood, urine and sweat and are vital to specific processes. Define Electrode Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Define Electrode Electrolyte To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and. Electrode and electrolyte are two essential components in electrochemical systems. Pretty much any dissociation into ions between 0% and 100% makes a chemical a weak electrolyte, but in practice,. Positively charged ions are called cations. An electrolyte is a. Define Electrode Electrolyte.