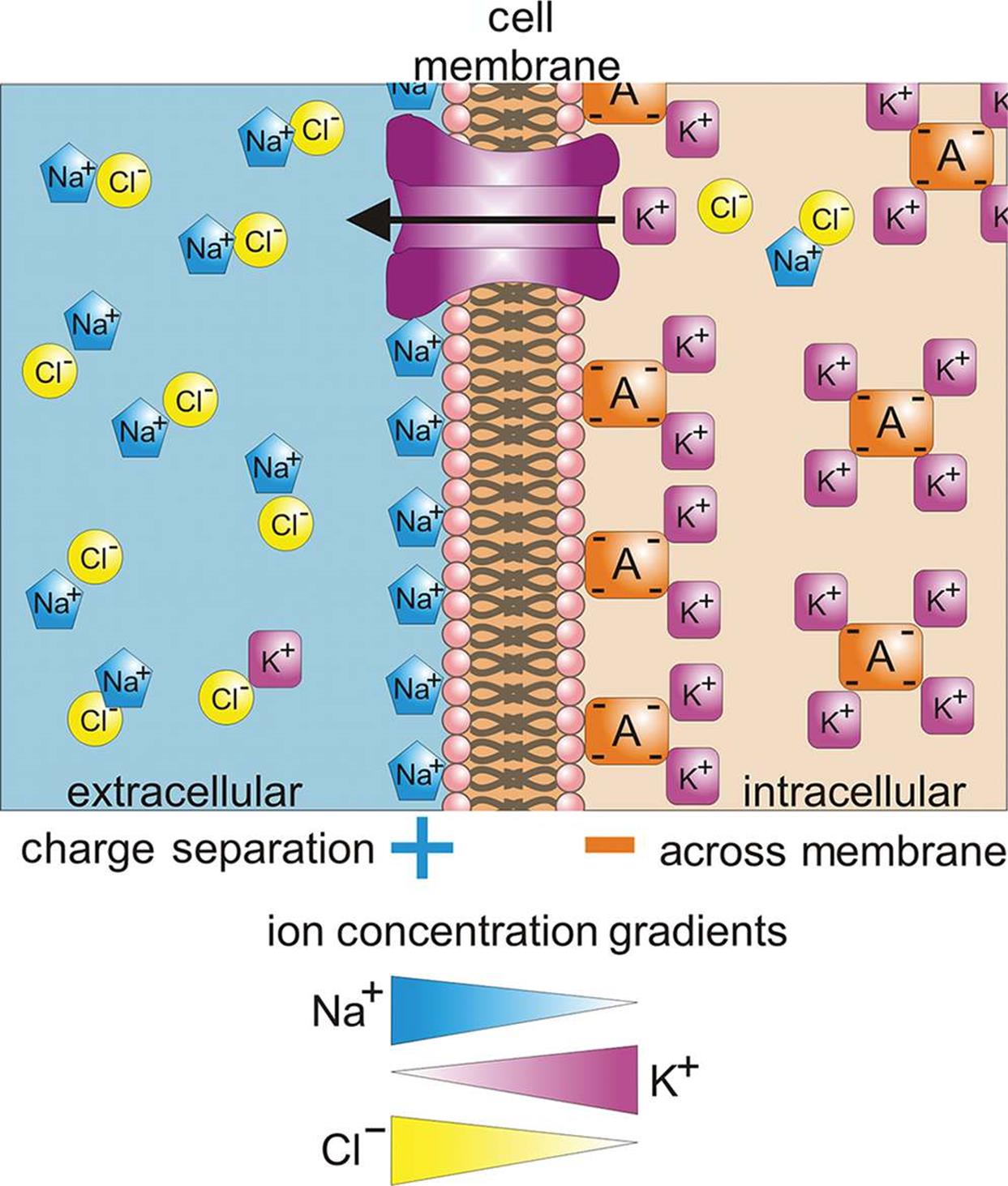
What Is Meant By Electrochemical Gradient . The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. These two forces working together are called an electrochemical gradient. The electrochemical gradient determines the. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and.
from schoolbag.info
The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. These two forces working together are called an electrochemical gradient. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and.
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell The electrochemical
What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. These two forces working together are called an electrochemical gradient. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation ID6600057 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the. The electrochemical gradient can be. What Is Meant By Electrochemical Gradient.
From www.squidinkillustration.com
Electrochemical Gradient — Squid Ink Illustration What Is Meant By Electrochemical Gradient The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the direction an ion moves by diffusion or active. What Is Meant By Electrochemical Gradient.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell The electrochemical What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID4723643 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. An electrochemical gradient is a difference in concentration and electric. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Membrane Structure and Function PowerPoint Presentation, free download ID825867 What Is Meant By Electrochemical Gradient The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. These two forces working together are called an electrochemical gradient. The electrochemical gradient determines. What Is Meant By Electrochemical Gradient.
From www.researchgate.net
Electrochemical gradient formation in Oenococcus oeni towards the... Download Scientific Diagram What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the. These two forces working together are called an electrochemical gradient. The electrical gradient. What Is Meant By Electrochemical Gradient.
From ditki.com
Electrochemical Gradient ditki medical and biological sciences What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient can be. What Is Meant By Electrochemical Gradient.
From www.youtube.com
Electrochemical Gradient and the Electron Transport Chain.mp4 YouTube What Is Meant By Electrochemical Gradient The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. These two forces working together are called an electrochemical gradient. The electrochemical gradient determines the. An electrochemical. What Is Meant By Electrochemical Gradient.
From alg.manifoldapp.org
“Chapter 8 The Electrochemical Gradient” in “Fundamentals of Cell Biology” on OpenALG What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient can be described as a driving force, which corresponds to. What Is Meant By Electrochemical Gradient.
From www.numerade.com
SOLVED what is meant by the term "membrane potential "? what is an electrochemical gradient? What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient determines the. The electrochemical gradient can be described as a driving force, which corresponds to. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation ID6600057 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane. What Is Meant By Electrochemical Gradient.
From quizlet.com
Electrochemical gradient across a membrane equation Diagram Quizlet What Is Meant By Electrochemical Gradient These two forces working together are called an electrochemical gradient. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient determines the. An electrochemical. What Is Meant By Electrochemical Gradient.
From alg.manifoldapp.org
“Chapter 8 The Electrochemical Gradient” in “Fundamentals of Cell Biology” on OpenALG What Is Meant By Electrochemical Gradient The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. The electrochemical gradient determines the direction that ions will flow through an. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT History of Fluid Mosaic Model PowerPoint Presentation, free download ID442932 What Is Meant By Electrochemical Gradient The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. These two forces working together are called an electrochemical gradient. The electrical gradient of k +, a positive ion, also tends. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Membrane Transport and Membrane Potentials PowerPoint Presentation ID418941 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. These two forces working together are called an electrochemical gradient. The electrochemical gradient. What Is Meant By Electrochemical Gradient.
From www.chegg.com
Solved Electrochemical gradient is the driving force for ion What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. These two forces working together are called an electrochemical gradient. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. The electrochemical. What Is Meant By Electrochemical Gradient.
From openbooks.lib.msu.edu
Membrane Potential Introduction to Neuroscience What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. These two forces working together are called an electrochemical gradient. The electrochemical gradient can be described as a driving force, which corresponds to the difference. What Is Meant By Electrochemical Gradient.
From www.jove.com
Gradient Protocols and Video Articles What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. These two forces working together are called an electrochemical gradient. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive.. What Is Meant By Electrochemical Gradient.
From www.youtube.com
Electrochemical Gradients and Membrane Transport (BIOS 041) YouTube What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient can be described as a driving force, which corresponds to. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation ID549038 What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the. These two forces working together are called an electrochemical. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Nerve physiology PowerPoint Presentation, free download ID4506197 What Is Meant By Electrochemical Gradient The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Lecture 4 BIO 344 PowerPoint Presentation, free download ID1451459 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient determines the. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. These two forces working together are called an electrochemical gradient. An electrochemical. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Electron Transport Chain PowerPoint Presentation, free download ID6588926 What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient. What Is Meant By Electrochemical Gradient.
From www.youtube.com
Electrochemical Gradient YouTube What Is Meant By Electrochemical Gradient These two forces working together are called an electrochemical gradient. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrochemical gradient. What Is Meant By Electrochemical Gradient.
From www.numerade.com
SOLVED OUTSIDE ++++ ++++ ++++ ++++ INSIDE concentration gradient (with no membrane potential What Is Meant By Electrochemical Gradient The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrochemical gradient determines the. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a.. What Is Meant By Electrochemical Gradient.
From slideplayer.com
What is a concentration gradient? ppt download What Is Meant By Electrochemical Gradient These two forces working together are called an electrochemical gradient. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction an ion moves by diffusion. What Is Meant By Electrochemical Gradient.
From quizlet.com
Electrochemical Gradient Diagram Quizlet What Is Meant By Electrochemical Gradient The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. These two forces working together are called an electrochemical gradient. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradient determines the direction an. What Is Meant By Electrochemical Gradient.
From slideplayer.com
Chapter 7 Membranes. ppt download What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient can be described as a driving force, which corresponds to. What Is Meant By Electrochemical Gradient.
From www.drawittoknowit.com
General Biology Glossary Electrochemical Gradient Draw It to Know It What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. These two forces working together are called an electrochemical gradient. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Chapter 7 Membrane Structure and Function PowerPoint Presentation ID1348372 What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the direction that ions will flow through an open ion. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Changes in electrical gradients PowerPoint Presentation, free download ID1756879 What Is Meant By Electrochemical Gradient The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Skeletal Muscle Physiology PowerPoint Presentation, free download ID1829145 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient can be described as a driving force, which corresponds to the difference between the membrane potential and. The electrochemical gradient determines the direction that ions. What Is Meant By Electrochemical Gradient.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID549548 What Is Meant By Electrochemical Gradient The electrochemical gradient determines the direction an ion moves by diffusion or active transport across a. The electrochemical gradient determines the. An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of. What Is Meant By Electrochemical Gradient.
From www.youtube.com
Concentration Gradients VS Electrochemical Gradients With Examples YouTube What Is Meant By Electrochemical Gradient An electrochemical gradient is a difference in concentration and electric charge across a membrane, which influences the movement of. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but. What Is Meant By Electrochemical Gradient.
From www.youtube.com
What is an Electrochemical Gradient? Bio Video Textbooks Preview YouTube What Is Meant By Electrochemical Gradient The electrochemical gradient determines the. The electrical gradient of k +, a positive ion, also tends to drive it into the cell, but the concentration gradient of k + tends to drive. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. These two forces working together. What Is Meant By Electrochemical Gradient.