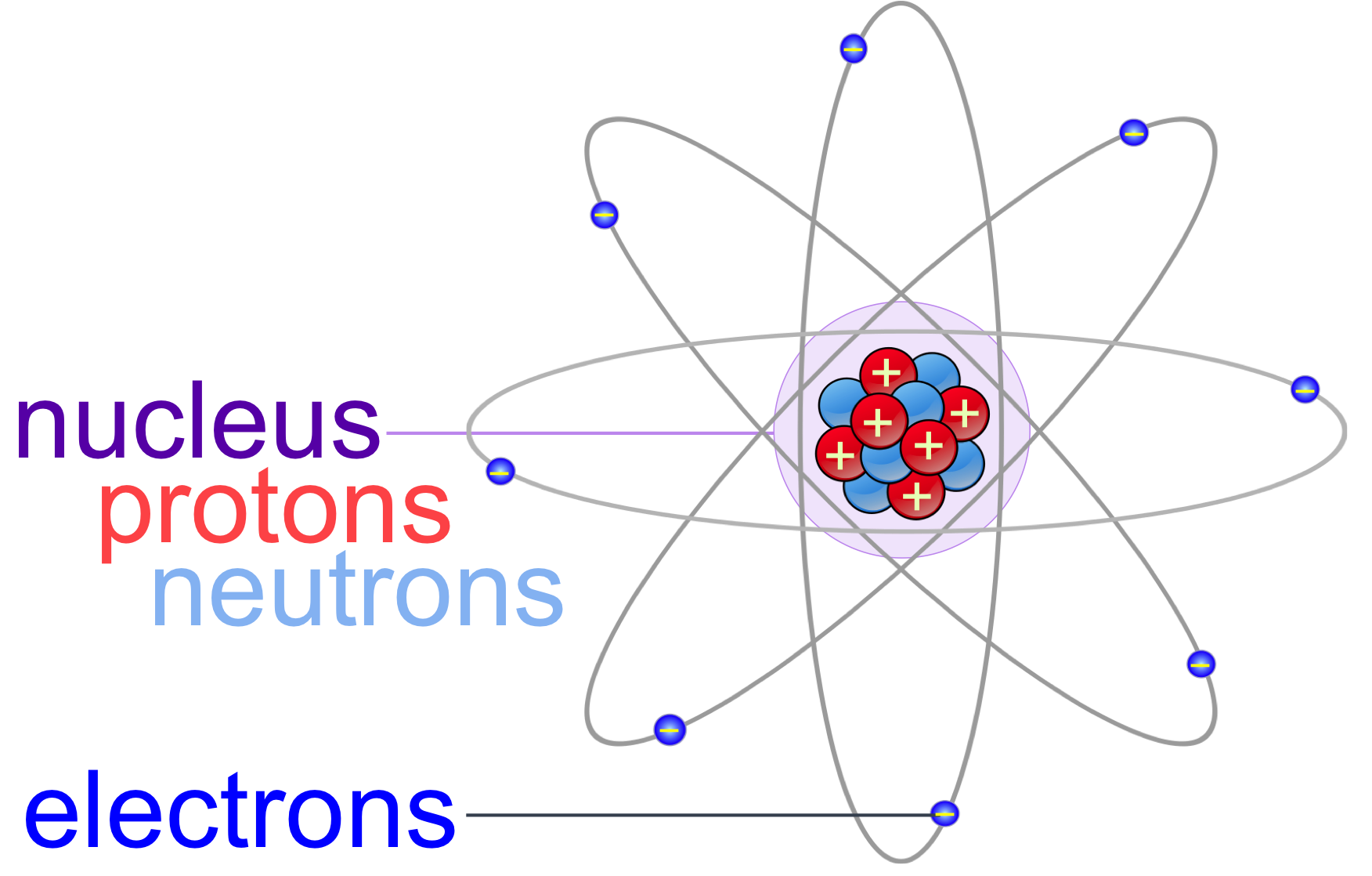
Do Ions Have Neutrons . An ion has an unequal number of protons and electrons. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. If the charge is positive, there are more protons than electrons. Determine the number of protons,. If the charge is negative, electrons are in. Atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes. Explain what isotopes are and how an isotope affects an element's atomic mass. Atoms are made up of protons, neutrons and electrons. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,.
from thebiologyprimer.com
Explain what isotopes are and how an isotope affects an element's atomic mass. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Determine the number of protons,. Atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes. If the charge is positive, there are more protons than electrons. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. If the charge is negative, electrons are in.
Atoms & Molecules echapter — The Biology Primer
Do Ions Have Neutrons The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. Explain what isotopes are and how an isotope affects an element's atomic mass. Change the number of neutrons in an atom and it becomes. If the charge is positive, there are more protons than electrons. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. If the charge is negative, electrons are in. Atoms are made up of protons, neutrons and electrons. Determine the number of protons,. An ion has an unequal number of protons and electrons. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Do Ions Have Neutrons Like cations, the charge on an anion is indicated using a superscript after a chemical formula. If the charge is negative, electrons are in. If the charge is positive, there are more protons than electrons. Atoms are made up of protons, neutrons and electrons. Neutrons are in every atom (with one exception), and they are bound together with other neutrons. Do Ions Have Neutrons.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. If the charge is negative, electrons are in. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Explain what isotopes are and how an isotope affects an element's atomic mass. If. Do Ions Have Neutrons.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by Do Ions Have Neutrons The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes. Change the number of neutrons. Do Ions Have Neutrons.
From www.nagwa.com
Question Video Determining the Number of Protons Neutrons and Do Ions Have Neutrons If the charge is negative, electrons are in. Change the number of neutrons in an atom and it becomes. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Explain what isotopes. Do Ions Have Neutrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. Determine the number of protons,. Change the number of neutrons in an atom and it becomes. Explain what isotopes are and how an isotope affects an element's atomic mass. If the charge is negative, electrons. Do Ions Have Neutrons.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Do Ions Have Neutrons Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. If the charge is negative, electrons are in. Determine the number of protons,. Explain what isotopes are and how an isotope affects an element's atomic mass. If the charge is positive, there are more protons than electrons. The. Do Ions Have Neutrons.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. If the charge is negative, electrons are in. Change the number of neutrons in an atom and it becomes. If the charge is positive, there are more protons than electrons. The number of neutrons is not a factor in whether an atom, functional group,. Do Ions Have Neutrons.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Do Ions Have Neutrons Change the number of neutrons in an atom and it becomes. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. An ion has an unequal number of protons. Do Ions Have Neutrons.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Do Ions Have Neutrons Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Explain what isotopes are and how an isotope affects an element's atomic mass. If the charge is positive, there are more protons than electrons. Neutrons are in every. Do Ions Have Neutrons.
From www.youtube.com
Number of Protons, Neutrons and Electrons of an Atom or Ion Chemistry Do Ions Have Neutrons Like cations, the charge on an anion is indicated using a superscript after a chemical formula. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. If the charge is negative, electrons are in. Determine the number of protons,. Change the number of neutrons in an atom and it becomes an. Do Ions Have Neutrons.
From www.youtube.com
Finding the Protons, Neutrons, Electrons, & Mass Number for Ions YouTube Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. If the charge is negative, electrons are in. If the charge is positive, there are more protons than electrons. Determine the number of protons,. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. The number of neutrons. Do Ions Have Neutrons.
From data.allenai.org
inside the atom (lesson 0772) TQA explorer Do Ions Have Neutrons An ion has an unequal number of protons and electrons. Change the number of neutrons in an atom and it becomes. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Atoms. Do Ions Have Neutrons.
From gageferscase.blogspot.com
9 Protons 10 Neutrons 10 Electrons Total Charge Do Ions Have Neutrons If the charge is positive, there are more protons than electrons. If the charge is negative, electrons are in. An ion has an unequal number of protons and electrons. Atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes. Atoms are made up of protons, neutrons and electrons. Explain what. Do Ions Have Neutrons.
From www.gauthmath.com
Solved How many protons, electron and neutron does bromine ion have Do Ions Have Neutrons Atoms are made up of protons, neutrons and electrons. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Explain what isotopes are and how an isotope affects an element's atomic mass. An ion has an unequal number of protons and electrons. If the charge is negative, electrons are in. Atoms are made up. Do Ions Have Neutrons.
From www.sliderbase.com
Formula of Ionic Compounds Do Ions Have Neutrons An ion has an unequal number of protons and electrons. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. If the charge is positive, there are more protons than electrons. The. Do Ions Have Neutrons.
From studypansophism.z21.web.core.windows.net
Which Element Has 15 Neutrons Do Ions Have Neutrons Atoms are made up of protons, neutrons and electrons. If the charge is positive, there are more protons than electrons. Atoms are made up of protons, neutrons and electrons. An ion has an unequal number of protons and electrons. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Neutrons are in every atom. Do Ions Have Neutrons.
From redesigngreece.blogspot.com
How To Find The Number Of Neutrons In An Element redesigngreece Do Ions Have Neutrons Change the number of neutrons in an atom and it becomes. Atoms are made up of protons, neutrons and electrons. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Determine the number of protons,. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion.. Do Ions Have Neutrons.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Ions Have Neutrons Determine the number of protons,. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. Change the number of neutrons in an atom and it becomes an isotope, change the number of. Do Ions Have Neutrons.
From h-o-m-e.org
16 Facts About The Neutrons Protons And Electrons Of Argon H.O.M.E. Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Determine the number of protons,. If the charge is negative, electrons are in. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Atoms are made up of protons, neutrons and electrons.. Do Ions Have Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Do Ions Have Neutrons Explain what isotopes are and how an isotope affects an element's atomic mass. If the charge is positive, there are more protons than electrons. Determine the number of protons,. Change the number of neutrons in an atom and it becomes. An ion has an unequal number of protons and electrons. Atoms are made up of protons, neutrons and electrons. Change. Do Ions Have Neutrons.
From azchemistry.com
Proton, Electron, Neutron Definition Formula Application Do Ions Have Neutrons Change the number of neutrons in an atom and it becomes. Explain what isotopes are and how an isotope affects an element's atomic mass. An ion has an unequal number of protons and electrons. If the charge is negative, electrons are in. Atoms are made up of protons, neutrons and electrons. The number of neutrons is not a factor in. Do Ions Have Neutrons.
From www.teachoo.com
Neutron Discovery, Difference and more Teachoo Concepts Do Ions Have Neutrons Atoms are made up of protons, neutrons and electrons. If the charge is positive, there are more protons than electrons. An ion has an unequal number of protons and electrons. Atoms are made up of protons, neutrons and electrons. Explain what isotopes are and how an isotope affects an element's atomic mass. The number of neutrons is not a factor. Do Ions Have Neutrons.
From www.youtube.com
2.1.5 Protons/neutrons/electrons in atoms/ions from mass/atomic number Do Ions Have Neutrons Atoms are made up of protons, neutrons and electrons. Explain what isotopes are and how an isotope affects an element's atomic mass. An ion has an unequal number of protons and electrons. Change the number of neutrons in an atom and it becomes. If the charge is positive, there are more protons than electrons. Scientists arbitrarily define this amount of. Do Ions Have Neutrons.
From www.slideserve.com
PPT How many protons , electrons, and neutrons are in an atom Do Ions Have Neutrons Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. If the charge is positive, there are more protons than electrons. Change the number of neutrons in an atom and it becomes. Determine the number of protons,. Like cations, the charge on an anion is indicated using a. Do Ions Have Neutrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Do Ions Have Neutrons If the charge is positive, there are more protons than electrons. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Determine the number of protons,. Change the number of neutrons in an atom and it becomes. Change the number of neutrons in an atom and it becomes an isotope, change. Do Ions Have Neutrons.
From slideplayer.com
Atomic Structure. ppt download Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. An ion has an unequal number of protons and electrons. Atoms are made. Do Ions Have Neutrons.
From www.unlimitededu.net
What is a neutron and its charge? Discovery and mass of a neutron Do Ions Have Neutrons If the charge is positive, there are more protons than electrons. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Explain what isotopes are and how an isotope affects an element's atomic mass. Like cations, the charge on an anion is indicated using a superscript after a. Do Ions Have Neutrons.
From courses.lumenlearning.com
Molecular and Ionic Compounds General Chemistry Do Ions Have Neutrons Explain what isotopes are and how an isotope affects an element's atomic mass. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. If the charge is negative, electrons are in. Determine. Do Ions Have Neutrons.
From www.learnatnoon.com
What is the Charge of a Neutron? Noon Academy Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Determine the number of protons,. Atoms are made up of protons, neutrons and electrons. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Neutrons are in every atom (with one exception), and they are bound together with. Do Ions Have Neutrons.
From www.worksheetsplanet.com
What is a Neutron Definition of Neutron Do Ions Have Neutrons Like cations, the charge on an anion is indicated using a superscript after a chemical formula. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Atoms are made up of protons, neutrons and electrons. Atoms are made up of protons, neutrons and electrons. If the charge is positive, there are. Do Ions Have Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Do Ions Have Neutrons Atoms are made up of protons, neutrons and electrons. If the charge is positive, there are more protons than electrons. The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. If the charge is negative,. Do Ions Have Neutrons.
From sciencenotes.org
What Is the Difference Between an Atom and an Ion? Do Ions Have Neutrons Change the number of neutrons in an atom and it becomes. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Atoms are made up of protons, neutrons and electrons. Like cations,. Do Ions Have Neutrons.
From www.slideserve.com
PPT Atoms, ions & isotopes Click on mouse or press ‘Enter’ to begin Do Ions Have Neutrons Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one dalton,. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus. If the charge is negative, electrons are in. An ion has an unequal number of protons and electrons. If the charge is. Do Ions Have Neutrons.
From www.slideserve.com
PPT The nucleus (centre of an atom) contains protons and neutrons Do Ions Have Neutrons Like cations, the charge on an anion is indicated using a superscript after a chemical formula. If the charge is positive, there are more protons than electrons. Explain what isotopes are and how an isotope affects an element's atomic mass. Atoms are made up of protons, neutrons and electrons. Scientists arbitrarily define this amount of mass as one atomic mass. Do Ions Have Neutrons.
From www.slideserve.com
PPT AQA GCSE Physics 27 Nuclear Physics PowerPoint Presentation Do Ions Have Neutrons Determine the number of protons,. Explain what isotopes are and how an isotope affects an element's atomic mass. An ion has an unequal number of protons and electrons. Change the number of neutrons in an atom and it becomes. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. Atoms are made up of. Do Ions Have Neutrons.