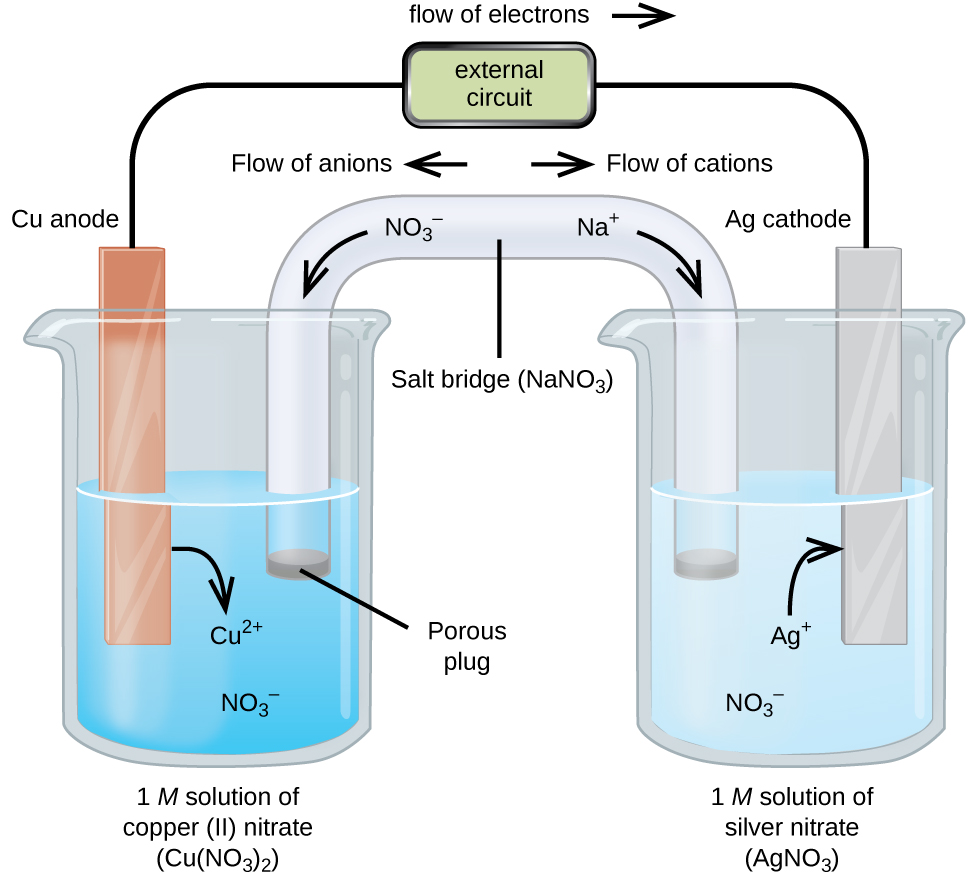
Example Electrochemical Analysis . We divide electrochemical techniques into static techniques and dynamic techniques. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Experienced experts provide the necessary. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. In a static technique we do not allow current to pass through the electrochemical. Ph measurement, ion selective electrodes, gas sensing electrodes. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems.
from alevelchemistry.co.uk
The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. We divide electrochemical techniques into static techniques and dynamic techniques. In a static technique we do not allow current to pass through the electrochemical. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. Experienced experts provide the necessary. Ph measurement, ion selective electrodes, gas sensing electrodes.
Electrochemical Cells Definition, Description & Types
Example Electrochemical Analysis Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. In a static technique we do not allow current to pass through the electrochemical. Experienced experts provide the necessary. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Ph measurement, ion selective electrodes, gas sensing electrodes. We divide electrochemical techniques into static techniques and dynamic techniques. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry.
From www.slideserve.com
PPT Chapter 2 Electrochemical Analysis PowerPoint Presentation, free Example Electrochemical Analysis In a static technique we do not allow current to pass through the electrochemical. Experienced experts provide the necessary. We divide electrochemical techniques into static techniques and dynamic techniques. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical,. Example Electrochemical Analysis.
From www.slideserve.com
PPT Electrochemical Analysis PowerPoint Presentation, free download Example Electrochemical Analysis An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. In a static technique we do not allow current to pass through the electrochemical. We divide electrochemical techniques into static techniques and dynamic techniques. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. In this chapter we turn our attention. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of (A) cyclic voltammogram of Example Electrochemical Analysis Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Experienced experts provide the necessary. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. We divide electrochemical techniques into static techniques and dynamic techniques. The. Example Electrochemical Analysis.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Experienced experts provide the necessary. We divide electrochemical techniques into static techniques and dynamic techniques. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. An overview of the electroanalytical techniques, such as cyclic voltammetry. Example Electrochemical Analysis.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Example Electrochemical Analysis In a static technique we do not allow current to pass through the electrochemical. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse. Example Electrochemical Analysis.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Experienced experts provide the necessary. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Ph measurement, ion selective electrodes, gas sensing electrodes. Electroanalytical techniques are. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis a cyclic voltammetry; b chronopotentiometry; c Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. We divide electrochemical techniques into static techniques and dynamic techniques. In. Example Electrochemical Analysis.
From www.slideserve.com
PPT Electrochemical methods of analysis PowerPoint Presentation, free Example Electrochemical Analysis Experienced experts provide the necessary. In a static technique we do not allow current to pass through the electrochemical. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the.. Example Electrochemical Analysis.
From www.researchgate.net
Examples of common electrochemical analysis schemes relevant to the Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. In a static technique we do not allow current to pass through the electrochemical. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. Electrochemical measurements are made in an electrochemical cell that consists of two. Example Electrochemical Analysis.
From www.slideshare.net
Electrochemical methods Environmental Analysis Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. In a static technique we do not allow current to pass through the electrochemical. We divide electrochemical techniques into static techniques and dynamic techniques. Experienced. Example Electrochemical Analysis.
From www.scribd.com
Electrochemical Analysis (Judy) Electrochemistry Example Electrochemical Analysis An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. We divide electrochemical techniques into static techniques and dynamic techniques. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to. Example Electrochemical Analysis.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Example Electrochemical Analysis Ph measurement, ion selective electrodes, gas sensing electrodes. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. In a static technique we do not allow current to pass through the electrochemical. In this chapter we turn our attention to electrochemical techniques in which the. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of Ni(OH)2 for OER processes. a Cyclic Example Electrochemical Analysis In a static technique we do not allow current to pass through the electrochemical. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Ph measurement, ion selective electrodes, gas sensing electrodes. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical,. Example Electrochemical Analysis.
From www.youtube.com
[Ch 1.4] Classification of Electrochemical Techniques YouTube Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. In a static technique we do not allow current to pass through the electrochemical. We divide electrochemical techniques into static techniques and dynamic techniques. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. The. Example Electrochemical Analysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. In a static technique we do not allow current to pass through the electrochemical. We divide electrochemical techniques into static techniques and dynamic techniques. Experienced experts provide the necessary. Ph measurement, ion selective electrodes, gas sensing electrodes. Electrochemical measurements are made in an electrochemical. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis in both 1.0 M KOH and 0.5 M H 2 SO 4 . a LSV Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. We divide electrochemical techniques into static techniques and dynamic techniques. In a static technique we. Example Electrochemical Analysis.
From www.slideserve.com
PPT Chapter 6 Electrochemical Analysis PowerPoint Presentation, free Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Experienced experts provide the necessary. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Ph measurement, ion selective electrodes, gas sensing electrodes. An overview of the electroanalytical techniques,. Example Electrochemical Analysis.
From blog.syrris.com
Electrochemistry made easy with continuous flow chemistry techniques Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. We divide electrochemical techniques into static techniques and dynamic techniques. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Experienced. Example Electrochemical Analysis.
From www.slideshare.net
Electrochemical method of analysis Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Ph measurement, ion selective electrodes, gas sensing electrodes. Experienced experts provide the necessary. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of Co/rGO composites synthesized via Example Electrochemical Analysis The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Experienced experts provide the necessary. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. In this chapter we turn our attention to electrochemical techniques. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of Rho cells collected from the treatments Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Experienced experts provide the necessary. We divide electrochemical techniques into static techniques and dynamic techniques. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of Pt and Pt/AuNP electrodes. (A) CVs of Pt Example Electrochemical Analysis Ph measurement, ion selective electrodes, gas sensing electrodes. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. We divide electrochemical techniques into static techniques and dynamic techniques. Experienced experts provide the necessary. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Electrochemical measurements are. Example Electrochemical Analysis.
From printorders.aip.org
Multiscale Modeling of Electrochemical Reactions and Processes AIP Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Experienced experts provide the necessary. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry.. Example Electrochemical Analysis.
From www.slideserve.com
PPT Electrochemical Analysis PowerPoint Presentation, free download Example Electrochemical Analysis We divide electrochemical techniques into static techniques and dynamic techniques. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. Experienced experts provide the necessary. In a static technique we do not allow current to pass through the electrochemical. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the. Example Electrochemical Analysis.
From ivypanda.com
Electrochemical Methods of Analysis 632 Words Case Study Example Example Electrochemical Analysis We divide electrochemical techniques into static techniques and dynamic techniques. In a static technique we do not allow current to pass through the electrochemical. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. The major electroanalytical methods include. Example Electrochemical Analysis.
From saylordotorg.github.io
Electrochemistry Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the.. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis. (a) Cyclic voltammogram (CV) of the cathode Example Electrochemical Analysis Experienced experts provide the necessary. We divide electrochemical techniques into static techniques and dynamic techniques. In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. In a static technique we do not allow current to pass through the electrochemical. Ph measurement, ion selective electrodes, gas sensing electrodes. The major electroanalytical methods. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of the CipTob oxidation process. (A) Cyclic Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Ph measurement, ion selective electrodes, gas sensing electrodes. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. In a static technique we do not allow current to pass through the electrochemical. Electroanalytical techniques are particularly useful. Example Electrochemical Analysis.
From dokumen.tips
(PPT) Chapter 6 electrochemical analysis DOKUMEN.TIPS Example Electrochemical Analysis An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. We divide electrochemical techniques into static techniques and dynamic techniques. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis. Example Electrochemical Analysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Example Electrochemical Analysis An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. In a static technique we do not allow current to pass through the electrochemical. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of FCNTMnO2 in a twocell configuration with Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. Experienced experts provide the necessary. Ph measurement, ion selective electrodes, gas sensing electrodes. We divide electrochemical techniques into static techniques and dynamic techniques. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. In a static technique. Example Electrochemical Analysis.
From www.researchgate.net
Electrochemical analysis of materials obtained using KOH activation at Example Electrochemical Analysis The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. Experienced experts provide the necessary. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry needed to control and measure the.. Example Electrochemical Analysis.
From www.pinterest.com
Electrochemical Cells Chemwiki Chemistry Textbook, Gcse Chemistry Example Electrochemical Analysis In this chapter we turn our attention to electrochemical techniques in which the potential, current, or charge in an. We divide electrochemical techniques into static techniques and dynamic techniques. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. In a static technique we do not allow current to pass through the electrochemical. Electroanalytical techniques are. Example Electrochemical Analysis.
From www.slideserve.com
PPT ELECTROCHEMICAL METHODS OF ANALYSIS 7 th lecture PowerPoint Example Electrochemical Analysis In a static technique we do not allow current to pass through the electrochemical. Experienced experts provide the necessary. Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. The major electroanalytical methods include potentiometry, amperometry, conductometry, electrogravimetry, voltammetry (and polarography), and coulometry. We divide electrochemical techniques into static techniques and dynamic techniques. Ph. Example Electrochemical Analysis.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Example Electrochemical Analysis Electroanalytical techniques are particularly useful for qualitative and quantitative analysis of chemical, biochemical, and physical systems. We divide electrochemical techniques into static techniques and dynamic techniques. An overview of the electroanalytical techniques, such as cyclic voltammetry (cv), differential pulse voltammetry (dpv),. Electrochemical measurements are made in an electrochemical cell that consists of two or more electrodes and the electronic circuitry. Example Electrochemical Analysis.