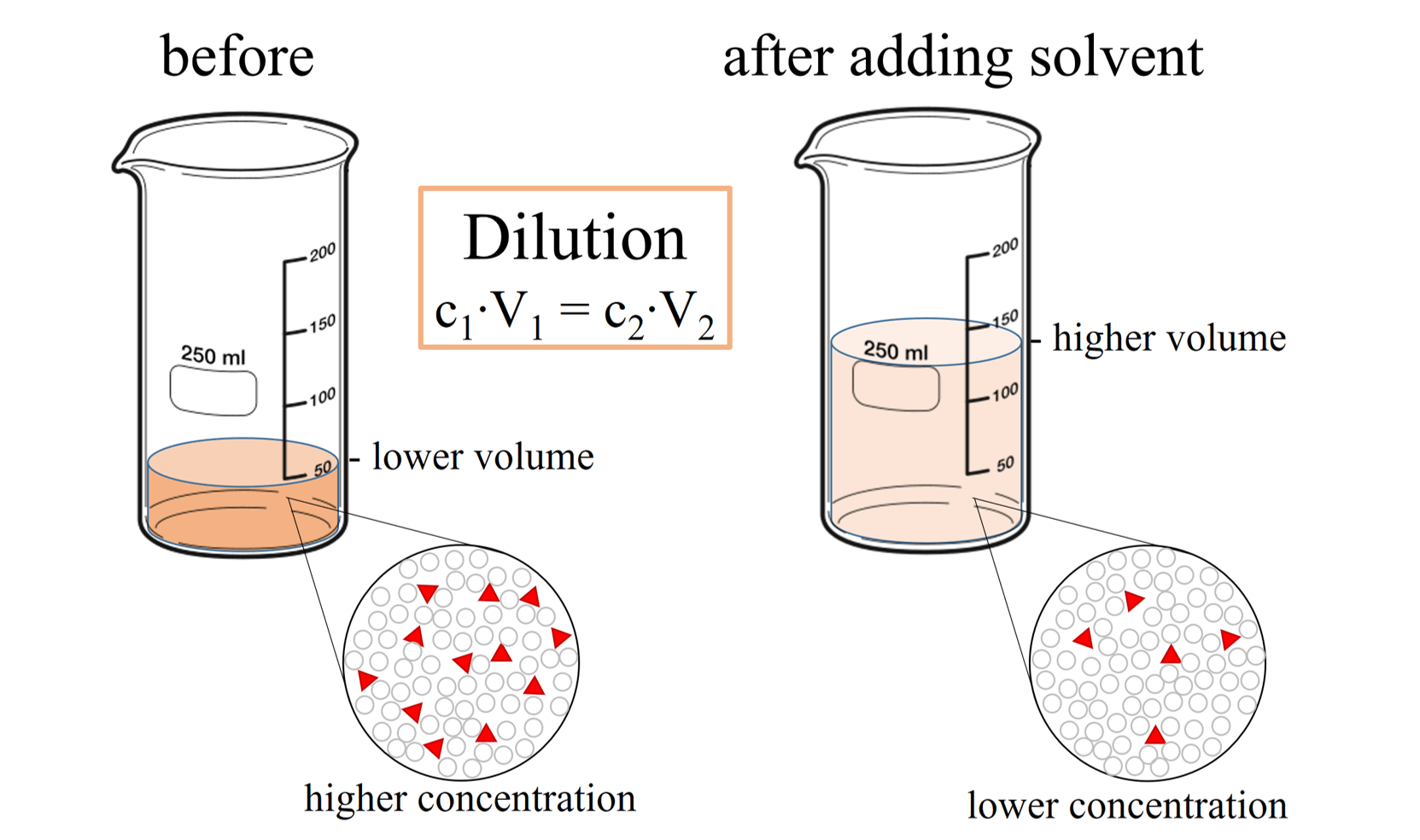
Dilution Questions Chemistry . Learn how to dilute and concentrate solutions. I add 560 ml more water to it? We are often concerned with how much solute is dissolved in a given amount of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. 10.0 ml of 1.00 m hcl is. Dilution is the addition of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution?
from quizizz.com
Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. We are often concerned with how much solute is dissolved in a given amount of. 10.0 ml of 1.00 m hcl is. There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
Dilutions / Pengenceran 51 plays Quizizz
Dilution Questions Chemistry We are often concerned with how much solute is dissolved in a given amount of. Learn how to dilute and concentrate solutions. 10.0 ml of 1.00 m hcl is. We are often concerned with how much solute is dissolved in a given amount of. There’s a bottle of 0.750 m nacl on a shelf. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution is the addition of. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution?
From www.youtube.com
Molarity Dilution Practice Problem 3 YouTube Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 10.0 ml of 1.00 m hcl is. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Explain what changes and what stays the same when 1.00 l of. Dilution Questions Chemistry.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Foundation Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Learn how to dilute and concentrate solutions. Dilution is the addition of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in. Dilution Questions Chemistry.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. 10.0 ml of 1.00 m hcl is. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilution Questions Chemistry.
From www.studypool.com
SOLUTION Dilution Problems Molarity Formula and Dilution Factor PAper Studypool Dilution Questions Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by changing the amount. Dilution Questions Chemistry.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube Dilution Questions Chemistry Understand how stock solutions are used in the laboratory. Dilution is the addition of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Explain what changes and what stays. Dilution Questions Chemistry.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Questions Chemistry Dilution is the addition of. 10.0 ml of 1.00 m hcl is. Learn how to dilute and concentrate solutions. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in. Dilution Questions Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Questions Chemistry How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Learn how to dilute and concentrate solutions. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We are often concerned with how much solute is dissolved in a given. Dilution Questions Chemistry.
From studylib.net
Dilution Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Learn how to dilute. Dilution Questions Chemistry.
From www.pinterest.com
Solutions and Molarity Quiz (Solutes, Solvents, Saturation, and Dilution) Physical science Dilution Questions Chemistry How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. A dilution is a process where the concentration of a solution is lowered by adding solvent to. Dilution Questions Chemistry.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Questions Chemistry 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. 1) if i have 340 ml of a 0.5 m nabr. Dilution Questions Chemistry.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Questions Chemistry I add 560 ml more water to it? Understand how stock solutions are used in the laboratory. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilution Questions Chemistry.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry CLEAR & SIMPLE Chemistry Dilution Questions Chemistry Understand how stock solutions are used in the laboratory. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. We are often concerned with how much solute is dissolved in a given amount of. 10.0 ml of 1.00 m hcl is. Often, a worker will need to change the concentration of a solution by. Dilution Questions Chemistry.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Questions Chemistry 10.0 ml of 1.00 m hcl is. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Understand how stock solutions are used in the laboratory. Often, a worker. Dilution Questions Chemistry.
From quizizz.com
Dilutions / Pengenceran 51 plays Quizizz Dilution Questions Chemistry We are often concerned with how much solute is dissolved in a given amount of. There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of. Understand how stock solutions are used in the laboratory. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? A dilution is a. Dilution Questions Chemistry.
From studylib.net
Key DilutionsWorksheet Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Often, a worker will need to change the concentration of a solution by changing the amount of solvent. I add 560 ml more water to it? A dilution is a process where the. Dilution Questions Chemistry.
From www.youtube.com
Testing the validity of Ostwald’s dilution law/Determination of dissociation constant of Acetic Dilution Questions Chemistry I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l.. Dilution Questions Chemistry.
From worksheets.clipart-library.com
Dilutions Worksheet Worksheets Library Dilution Questions Chemistry 10.0 ml of 1.00 m hcl is. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution is the addition of. Understand how stock solutions are used in the laboratory. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? I add 560 ml. Dilution Questions Chemistry.
From www.youtube.com
Serial Dilution Question General Chemistry YouTube Dilution Questions Chemistry Often, a worker will need to change the concentration of a solution by changing the amount of solvent. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. There’s a bottle of. Dilution Questions Chemistry.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Questions Chemistry 10.0 ml of 1.00 m hcl is. There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of. Learn how to dilute and concentrate solutions. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Often, a worker will need to change the concentration of a solution by. Dilution Questions Chemistry.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Questions Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by changing the amount. Dilution Questions Chemistry.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? Learn how to dilute and concentrate solutions. 10.0 ml of 1.00 m hcl is. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. 1) if i have 340 ml of. Dilution Questions Chemistry.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use the following example of a Dilution Questions Chemistry Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. I add. Dilution Questions Chemistry.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 10.0 ml of 1.00 m hcl is. Dilution is the addition of. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. We are often concerned with how much solute is dissolved in a given amount. Dilution Questions Chemistry.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Questions Chemistry We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. There’s a bottle of 0.750 m nacl on a shelf. 10.0 ml of 1.00 m hcl is. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Explain. Dilution Questions Chemistry.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Questions Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? We are often concerned with how much solute is dissolved in a. Dilution Questions Chemistry.
From study.com
Quiz & Worksheet How to Calculate Dilution of Solutions Dilution Questions Chemistry Understand how stock solutions are used in the laboratory. Learn how to dilute and concentrate solutions. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. How much of it do. Dilution Questions Chemistry.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Questions Chemistry Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. 10.0 ml of 1.00 m hcl is. Dilution is the addition of. Explain what changes and what stays the same when 1.00 l of a solution of nacl. Dilution Questions Chemistry.
From studylib.net
Dilutions Worksheet Dilution Questions Chemistry A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with how much solute is dissolved in a given amount of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute. Dilution Questions Chemistry.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations YouTube Dilution Questions Chemistry Dilution is the addition of. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 10.0 ml of 1.00 m hcl is. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilution Questions Chemistry.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Questions Chemistry Understand how stock solutions are used in the laboratory. I add 560 ml more water to it? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80. Dilution Questions Chemistry.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Formula & Equation Stock Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Understand how stock solutions are used in the laboratory. 10.0 ml of 1.00 m hcl is. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Often, a worker will need. Dilution Questions Chemistry.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 10.0 ml of 1.00 m hcl is. Understand how stock solutions are used in the laboratory. Dilution is the addition of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l.. Dilution Questions Chemistry.
From worksheetkrause.z19.web.core.windows.net
Dilution Problems Chemistry Worksheet Dilution Questions Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution is the addition of. How much of it do you need to prepare 50 ml of a 0.10 m nacl solution? Understand how stock solutions are used in the laboratory. I add 560 ml more water to it? 2) if i. Dilution Questions Chemistry.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. How much of it do you need to prepare 50 ml of a. Dilution Questions Chemistry.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Questions Chemistry There’s a bottle of 0.750 m nacl on a shelf. Learn how to dilute and concentrate solutions. 10.0 ml of 1.00 m hcl is. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Dilution Questions Chemistry.