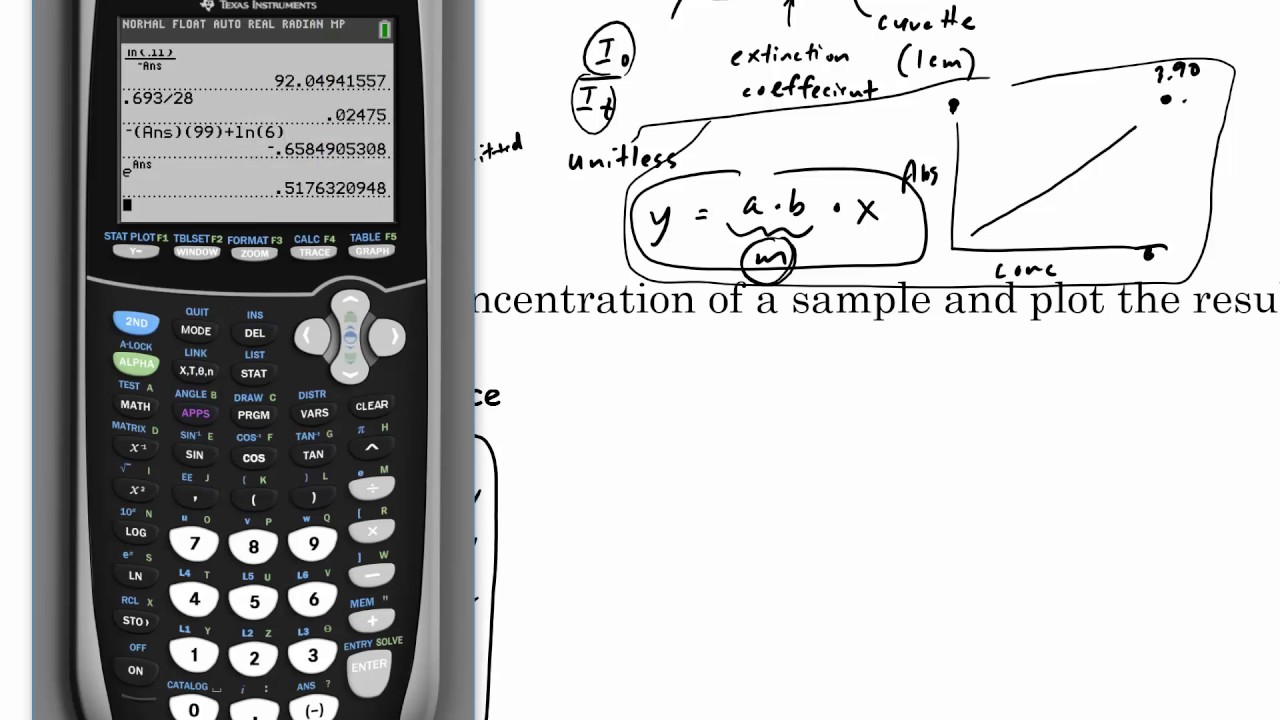
Spectrophotometer And Beer's Law . Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law.
from www.youtube.com
The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You can also use this calculator to determine the concentration of. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law.
Spectrophotometer and Beer's Law YouTube
Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and.
From www.chegg.com
The Use of the Spectrophotometer and Beer's Law Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a. Spectrophotometer And Beer's Law.
From www.youtube.com
How to compute for the concentration of the unknown? Spectrophotometer, Beer's Law & Absorbance Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship. Spectrophotometer And Beer's Law.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometer and Beer's Law YouTube Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Spectrophotometer And Beer's Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometer And Beer's Law.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law is a mathematical equation relating the absorbance ( a) of a. Spectrophotometer And Beer's Law.
From www.youtube.com
Use of spectrophotometer and Beer’s Law to determine unknown protein concentrations YouTube Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You can also use this calculator to determine the concentration of. Explore. Spectrophotometer And Beer's Law.
From icuvets.com
UV Visible Spectrophotometer Setup And BeerLambert Law ICuvets Cells Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. This. Spectrophotometer And Beer's Law.
From www.youtube.com
spectrum, UV Visible Spectroscopy, Beer Lambert’s Law, Electronic Transitions Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Spectrophotometer And Beer's Law Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. You can also use this calculator to determine the concentration of. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law is a mathematical. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometers, calibration curves and Beer's Law YouTube Spectrophotometer And Beer's Law This relationship is called the. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Spectrophotometer And Beer's Law.
From glossary.periodni.com
Beer’s law Chemistry Dictionary & Glossary Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You can also use this calculator to determine the concentration of. This relationship is called the. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. The amount of light that a species. Spectrophotometer And Beer's Law.
From www.studocu.com
Spectophotometer and Beers Law Lab Report Introduction to Spectrophotometer and Beer’s Law Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all. Spectrophotometer And Beer's Law.
From www.studypool.com
SOLUTION Spectroscopy ans beer lamberts law and spectrum and energy level Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You can also use this calculator to determine the concentration of. Explore how beer's law. Spectrophotometer And Beer's Law.
From sciencenotes.org
Beer's Law Equation and Example Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship. Spectrophotometer And Beer's Law.
From www.studocu.com
Lab Report Spectrophotometer and Beer’s Law Graph 1 Madison McDaniel February 8th, 2022 CHEM Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You can also use this calculator to determine the concentration of. Since the concentration, path. Spectrophotometer And Beer's Law.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil HøjlundNielsen YouTube Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. Since the concentration, path length and molar absorptivity are all directly proportional. Spectrophotometer And Beer's Law.
From stock.adobe.com
schematic diagram of spectrophotometer, UV visible spectrophotometer, beer lambert law. chemical Spectrophotometer And Beer's Law Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. You can also use this calculator to determine the concentration of. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity. Spectrophotometer And Beer's Law.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. This relationship is called the. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the. Spectrophotometer And Beer's Law.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation, free download ID211563 Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. You can also use this calculator to determine the concentration of. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Since the concentration, path length and molar absorptivity are all directly proportional to. Spectrophotometer And Beer's Law.
From exoegsuog.blob.core.windows.net
BeerLambert Law Spectrophotometer Pdf at Kristin Wright blog Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. You can also use this calculator to determine the concentration of. Beer's law is a mathematical. Spectrophotometer And Beer's Law.
From www.studocu.com
Chemistry Lab 1 Report n/a Lab Report Introduction to the Spectrophotometer and Beer’s Law Spectrophotometer And Beer's Law Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometer And Beer's Law.
From www.thoughtco.com
Beer's Law Definition and Equation Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. This relationship is called the. The amount of light that a species. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Spectrophotometer And Beer's Law Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the. Spectrophotometer And Beer's Law.
From study.com
BeerLambert Law Equation, Units & Examples Lesson Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. You. Spectrophotometer And Beer's Law.
From www.youtube.com
BeerLambert's law with derivation/ Absorbance & Transmittance/ Spectrophotometer cell JAM CSIR Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. You. Spectrophotometer And Beer's Law.
From slidetodoc.com
Beers Lambert Law and Standard Curves BCH 312 Spectrophotometer And Beer's Law Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Spectrophotometer And Beer's Law You can also use this calculator to determine the concentration of. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule. Spectrophotometer And Beer's Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Since the concentration, path length and molar absorptivity are all. Spectrophotometer And Beer's Law.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore how beer's law is used in spectrophotometry and solve problems involving beer's law. Since. Spectrophotometer And Beer's Law.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometer And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Spectrophotometer And Beer's Law.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Spectrophotometer And Beer's Law This relationship is called the. Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all. Spectrophotometer And Beer's Law.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Spectrophotometer And Beer's Law Beer's law is a mathematical equation relating the absorbance ( a) of a solution to the molar concentration of the absorbing molecule and. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Spectrophotometer And Beer's Law.
From www.chegg.com
Spectrophotometry and Beer's Law spermanent Lab Spectrophotometer And Beer's Law This relationship is called the. You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Spectrophotometer And Beer's Law.