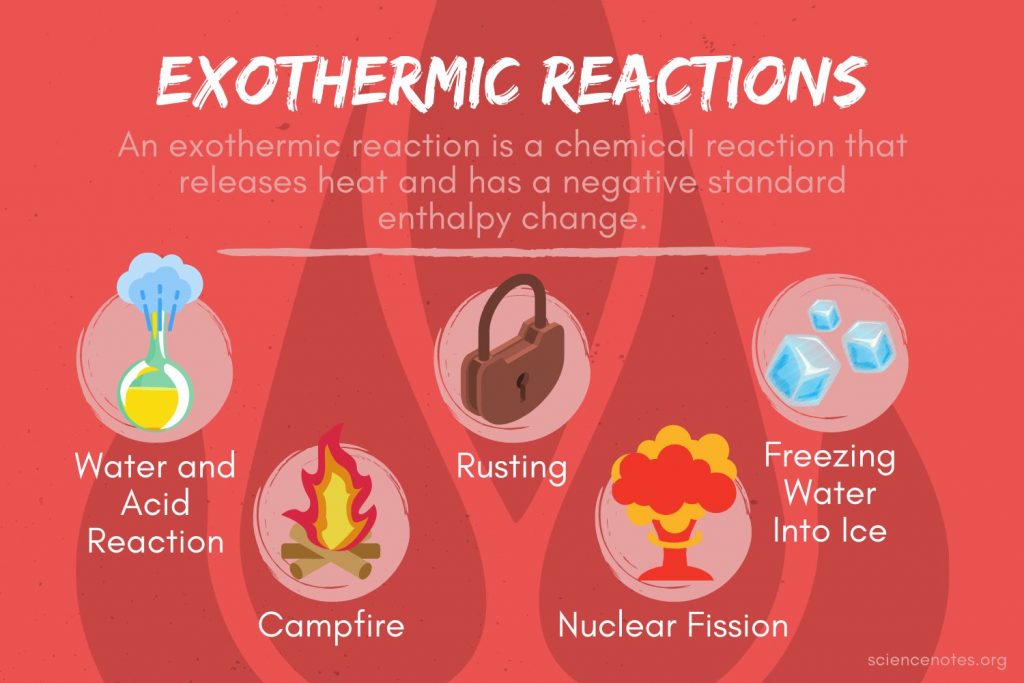
Burning Match Endothermic Or Exothermic . Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Energy changes are frequently shown by drawing an energy diagram. To answer this, we need to. The sign of \ (q\) for an endothermic process is positive because the. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Define endothermic and exothermic reactions. The ignition of the match causes a chemical. Read on to learn about how to. This means it releases heat to the surroundings. The quantity of heat for a process is represented by the letter \ (q\). Energy changes in chemical reactions. The formation of compounds from the constituent elements is almost always exothermic. One of these questions is whether the process of burning a match is endothermic or exothermic. Reactions can either generate or consume energy in the form of heat. Striking a match is an exothermic process.
from classnotes123.com
Read on to learn about how to. The quantity of heat for a process is represented by the letter \ (q\). One of these questions is whether the process of burning a match is endothermic or exothermic. Define endothermic and exothermic reactions. The formation of compounds from the constituent elements is almost always exothermic. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. This means it releases heat to the surroundings. Reactions can either generate or consume energy in the form of heat. To answer this, we need to. Striking a match is an exothermic process.
What does one mean by exothermic and endothermic reactions? Give
Burning Match Endothermic Or Exothermic Energy changes are frequently shown by drawing an energy diagram. One of these questions is whether the process of burning a match is endothermic or exothermic. This means it releases heat to the surroundings. The formation of compounds from the constituent elements is almost always exothermic. Read on to learn about how to. To answer this, we need to. Describe how heat is transferred in endothermic and exothermic reactions. Energy changes in chemical reactions. The sign of \ (q\) for an endothermic process is positive because the. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Striking a match is an exothermic process. Define endothermic and exothermic reactions. Reactions can either generate or consume energy in the form of heat. The ignition of the match causes a chemical.
From slideplayer.com
Physical vs. Chemical Changes ppt download Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. This means it releases heat to the surroundings. Define endothermic and exothermic reactions. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Describe how heat is transferred in endothermic and exothermic reactions. One of these questions is whether the process of burning a match is endothermic or. Burning Match Endothermic Or Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Burning Match Endothermic Or Exothermic Energy changes are frequently shown by drawing an energy diagram. Define endothermic and exothermic reactions. This means it releases heat to the surroundings. The ignition of the match causes a chemical. Read on to learn about how to. Reactions can either generate or consume energy in the form of heat. Energy changes in chemical reactions. The quantity of heat for. Burning Match Endothermic Or Exothermic.
From physicsinmyview.com
Difference between Endothermic and Exothermic Reaction Physics In My View Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter \ (q\). This means it releases heat to the surroundings. Formation of water from molecular hydrogen and oxygen and the formation of a metal. The ignition of the match causes a chemical. The formation of compounds from the. Burning Match Endothermic Or Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. This means it releases heat to the surroundings. The quantity of heat for a process is represented by the letter \ (q\). Striking a match is an exothermic process. Formation of water from molecular hydrogen and oxygen and the formation of a metal. The formation of compounds from the constituent elements is. Burning Match Endothermic Or Exothermic.
From www.chegg.com
Solved Classify the reactions as either endothermic or Burning Match Endothermic Or Exothermic This means it releases heat to the surroundings. The sign of \ (q\) for an endothermic process is positive because the. The ignition of the match causes a chemical. Striking a match is an exothermic process. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Energy changes are frequently shown by drawing an energy diagram.. Burning Match Endothermic Or Exothermic.
From giohpaiph.blob.core.windows.net
Is Candle Burning Exothermic Or Endothermic at Roy Grammer blog Burning Match Endothermic Or Exothermic One of these questions is whether the process of burning a match is endothermic or exothermic. The ignition of the match causes a chemical. To answer this, we need to. The sign of \ (q\) for an endothermic process is positive because the. Striking a match is an exothermic process. Reactions can either generate or consume energy in the form. Burning Match Endothermic Or Exothermic.
From schematiclistpact101.z22.web.core.windows.net
Potential Energy Diagram Exothermic Reaction Burning Match Endothermic Or Exothermic Energy changes are frequently shown by drawing an energy diagram. Describe how heat is transferred in endothermic and exothermic reactions. Reactions can either generate or consume energy in the form of heat. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. The ignition of the match causes a chemical. Energy changes in. Burning Match Endothermic Or Exothermic.
From slideplayer.com
Energy, Temperature, And Heat. ppt download Burning Match Endothermic Or Exothermic Reactions can either generate or consume energy in the form of heat. This means it releases heat to the surroundings. Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Describe how heat is transferred in endothermic and exothermic reactions. Striking a match. Burning Match Endothermic Or Exothermic.
From hxevvbgky.blob.core.windows.net
Endothermic Examples In Chemistry at Beverly Spain blog Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Striking a match is an exothermic process. The quantity of heat for a process is represented by the letter \ (q\). Formation of water from molecular hydrogen and oxygen and the formation of a metal. One of these questions is whether the process of burning a match is endothermic or. Burning Match Endothermic Or Exothermic.
From slideplayer.com
Energy, Temperature, And Heat. ppt download Burning Match Endothermic Or Exothermic The formation of compounds from the constituent elements is almost always exothermic. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. One of these questions is whether the process of burning a match is endothermic or exothermic. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy.. Burning Match Endothermic Or Exothermic.
From johnnycounterfit.com
Is Burning Wood Exothermic Or Endothermic Johnny Counterfit Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Striking a match is an exothermic process. This means it releases heat to the surroundings. Reactions can either generate or consume energy in the form of heat. Energy changes are frequently shown by drawing an energy diagram. Formation of water from molecular hydrogen and oxygen and the formation of a. Burning Match Endothermic Or Exothermic.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing Burning Match Endothermic Or Exothermic Formation of water from molecular hydrogen and oxygen and the formation of a metal. Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. One of these questions is whether the process of burning a match is endothermic or exothermic. Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden. Burning Match Endothermic Or Exothermic.
From slideplayer.com
Review. ppt download Burning Match Endothermic Or Exothermic This means it releases heat to the surroundings. The quantity of heat for a process is represented by the letter \ (q\). To answer this, we need to. Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Energy changes are. Burning Match Endothermic Or Exothermic.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Burning Match Endothermic Or Exothermic The quantity of heat for a process is represented by the letter \ (q\). The ignition of the match causes a chemical. To answer this, we need to. This means it releases heat to the surroundings. Read on to learn about how to. One of these questions is whether the process of burning a match is endothermic or exothermic. Striking. Burning Match Endothermic Or Exothermic.
From circuitestrilhatc6.z13.web.core.windows.net
Energy Diagram Chemdraw Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. Read on to learn about how to. This means it releases heat to the surroundings. Energy changes are frequently shown by drawing an energy diagram. To answer this, we need to. Describe how heat is transferred in endothermic and exothermic reactions. The sign of \ (q\) for an endothermic process is positive. Burning Match Endothermic Or Exothermic.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Burning Match Endothermic Or Exothermic Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Reactions can either generate or consume energy in the form of heat. Describe how heat is transferred in endothermic and exothermic reactions. Read on to learn about how to. Energy changes in chemical reactions. This means it releases heat to the surroundings. One. Burning Match Endothermic Or Exothermic.
From wordwall.net
Exothermic and endothermic definitions Match up Burning Match Endothermic Or Exothermic Striking a match is an exothermic process. Energy changes in chemical reactions. One of these questions is whether the process of burning a match is endothermic or exothermic. Read on to learn about how to. The sign of \ (q\) for an endothermic process is positive because the. This means it releases heat to the surroundings. Describe how heat is. Burning Match Endothermic Or Exothermic.
From a-z-animals.com
Endothermic vs. Exothermic Reactions Key Differences and Examples A Burning Match Endothermic Or Exothermic Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Energy changes are frequently shown by drawing an energy diagram. One of these questions is whether the process of burning a match is endothermic or exothermic. Reactions can either generate or consume energy in the form of heat. Striking a match is an. Burning Match Endothermic Or Exothermic.
From www.vrogue.co
Differentiate Between Exothermic And Endothermic Reac vrogue.co Burning Match Endothermic Or Exothermic Formation of water from molecular hydrogen and oxygen and the formation of a metal. Read on to learn about how to. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Describe how heat is transferred in endothermic and exothermic reactions. Reactions can either generate or consume energy in the form of heat.. Burning Match Endothermic Or Exothermic.
From brainly.com
Is a burning fire endothermic or exothermic how do you know Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. Describe how heat is transferred in endothermic and exothermic reactions. The formation of compounds from the constituent elements is almost always exothermic. To answer this, we need to. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Read on to learn about how to.. Burning Match Endothermic Or Exothermic.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. The formation of compounds from the constituent elements is almost always exothermic. The quantity of heat for a process is represented by the letter \ (q\). Read on to learn about how to. One of these questions is whether the process of burning a match is endothermic or exothermic. To answer this,. Burning Match Endothermic Or Exothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Burning Match Endothermic Or Exothermic Striking a match is an exothermic process. The formation of compounds from the constituent elements is almost always exothermic. The sign of \ (q\) for an endothermic process is positive because the. One of these questions is whether the process of burning a match is endothermic or exothermic. The ignition of the match causes a chemical. This means it releases. Burning Match Endothermic Or Exothermic.
From slideplayer.com
Atoms/Atomic Structure, PTE, Valence Electrons, Gravity, Bonding ppt Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Energy changes are frequently shown by drawing an energy diagram. One of these questions is whether the process of burning a match is endothermic or exothermic. This means. Burning Match Endothermic Or Exothermic.
From wordwall.net
Endothermic and exothermic reactions Match up Burning Match Endothermic Or Exothermic The quantity of heat for a process is represented by the letter \ (q\). Define endothermic and exothermic reactions. The formation of compounds from the constituent elements is almost always exothermic. To answer this, we need to. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Read on to learn about how. Burning Match Endothermic Or Exothermic.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Burning Match Endothermic Or Exothermic Formation of water from molecular hydrogen and oxygen and the formation of a metal. Energy changes are frequently shown by drawing an energy diagram. Energy changes in chemical reactions. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter \ (q\). One of these questions is whether the process of burning a match. Burning Match Endothermic Or Exothermic.
From in.pinterest.com
Learn about exothermic and endothermic reactions in this blog post here Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Formation of water from molecular hydrogen and oxygen and the formation of a metal. The formation of compounds from the constituent elements is almost always exothermic. This means it releases heat to the surroundings. The ignition of the match causes a chemical. Define endothermic and exothermic reactions. Striking a match. Burning Match Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Burning Match Endothermic Or Exothermic Energy changes are frequently shown by drawing an energy diagram. Reactions can either generate or consume energy in the form of heat. Define endothermic and exothermic reactions. Energy changes in chemical reactions. Describe how heat is transferred in endothermic and exothermic reactions. One of these questions is whether the process of burning a match is endothermic or exothermic. The quantity. Burning Match Endothermic Or Exothermic.
From slideplayer.com
Year 8 Chemical vs. Physical Changes ppt download Burning Match Endothermic Or Exothermic This means it releases heat to the surroundings. The quantity of heat for a process is represented by the letter \ (q\). The ignition of the match causes a chemical. Energy changes are frequently shown by drawing an energy diagram. To answer this, we need to. Describe how heat is transferred in endothermic and exothermic reactions. The formation of compounds. Burning Match Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. Energy changes are frequently shown by drawing an energy diagram. To answer this, we need to. This means it releases heat to the surroundings. The sign of \ (q\) for an endothermic process is positive because the. Reactions can either generate or consume energy in the form of heat. Energy diagrams show. Burning Match Endothermic Or Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Burning Match Endothermic Or Exothermic Energy changes in chemical reactions. The sign of \ (q\) for an endothermic process is positive because the. Define endothermic and exothermic reactions. The ignition of the match causes a chemical. Energy changes are frequently shown by drawing an energy diagram. Describe how heat is transferred in endothermic and exothermic reactions. The quantity of heat for a process is represented. Burning Match Endothermic Or Exothermic.
From fyoqnebwe.blob.core.windows.net
Is A Candle Burning Exothermic Or Endothermic at Lee Outlaw blog Burning Match Endothermic Or Exothermic The quantity of heat for a process is represented by the letter \ (q\). Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. The formation of compounds from the constituent elements is almost always exothermic. Energy changes in chemical reactions. Define endothermic and exothermic reactions. This means it releases heat to the. Burning Match Endothermic Or Exothermic.
From manuallistgelsemine.z13.web.core.windows.net
Exothermic And Endothermic Diagrams Burning Match Endothermic Or Exothermic The ignition of the match causes a chemical. Striking a match is an exothermic process. The formation of compounds from the constituent elements is almost always exothermic. Energy changes in chemical reactions. Formation of water from molecular hydrogen and oxygen and the formation of a metal. Energy changes are frequently shown by drawing an energy diagram. To answer this, we. Burning Match Endothermic Or Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Burning Match Endothermic Or Exothermic Define endothermic and exothermic reactions. One of these questions is whether the process of burning a match is endothermic or exothermic. The quantity of heat for a process is represented by the letter \ (q\). Describe how heat is transferred in endothermic and exothermic reactions. Energy changes are frequently shown by drawing an energy diagram. The sign of \ (q\). Burning Match Endothermic Or Exothermic.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Burning Match Endothermic Or Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Reactions can either generate or consume energy in the form of heat. Energy changes in chemical reactions. Energy diagrams show the stored/hidden energy of the reactants and products as well as the activation energy. Striking a match is an exothermic process. Energy changes are frequently shown by drawing an energy. Burning Match Endothermic Or Exothermic.
From www.youtube.com
Classify the following processes as exothermic or endothermic (A Burning Match Endothermic Or Exothermic Formation of water from molecular hydrogen and oxygen and the formation of a metal. Reactions can either generate or consume energy in the form of heat. To answer this, we need to. The quantity of heat for a process is represented by the letter \ (q\). Describe how heat is transferred in endothermic and exothermic reactions. Energy changes in chemical. Burning Match Endothermic Or Exothermic.