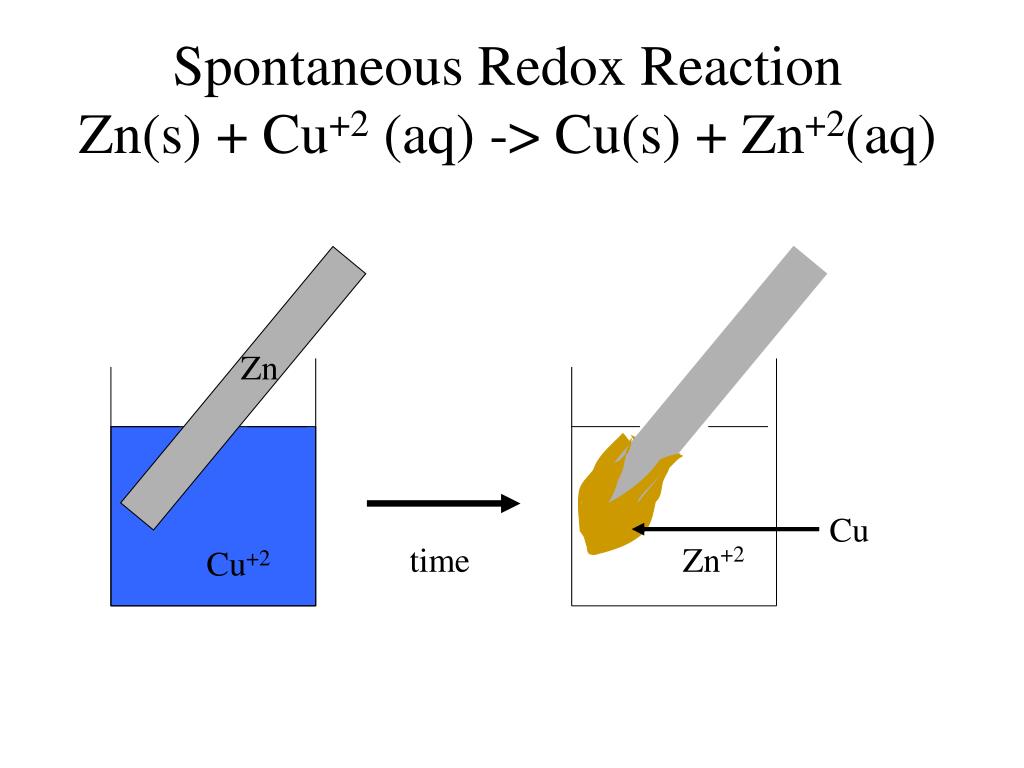
Electrochemical Cell Redox Reaction . an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Redox reactions are a major class of chemical reactions in which there is an exchange of. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions.
from www.slideserve.com
devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox.
PPT Redox Reactions and Electrochemistry PowerPoint Presentation
Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. Redox reactions are a major class of chemical reactions in which there is an exchange of.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Electrochemical Cell Redox Reaction if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. . Electrochemical Cell Redox Reaction.
From www.researchgate.net
Electrocatalytic redox reaction mechanism Download Scientific Diagram Electrochemical Cell Redox Reaction the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. Redox reactions are a major class of chemical reactions in which there is an exchange of. . Electrochemical Cell Redox Reaction.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemical Cell Redox Reaction a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. if a redox reaction can be split into half reactions it becomes possible to build a device, called. Electrochemical Cell Redox Reaction.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Electrochemical Cell Redox Reaction if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that. Electrochemical Cell Redox Reaction.
From www.researchgate.net
Redox reaction mechanism and electrochemical performance of full cells Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. an apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrochemical Cell Redox Reaction.
From www.youtube.com
Redox reaction in an electrolytic cell YouTube Electrochemical Cell Redox Reaction the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if. Electrochemical Cell Redox Reaction.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that. Electrochemical Cell Redox Reaction.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. if a redox reaction can be split into half reactions it becomes possible to build. Electrochemical Cell Redox Reaction.
From www.youtube.com
REDOX REACTIONS AND ELECTRODE PROCESSES YouTube Electrochemical Cell Redox Reaction if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. devices of this sort are generally referred to as electrochemical cells, and those in which. Electrochemical Cell Redox Reaction.
From www.researchgate.net
Schematic illustration of redox reaction mechanism in the presence of Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses. Electrochemical Cell Redox Reaction.
From studyschoolnucleator.z21.web.core.windows.net
Consider The Following Electrochemical Cell Electrochemical Cell Redox Reaction the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. devices of this sort are generally referred to as electrochemical cells, and those in which a. Electrochemical Cell Redox Reaction.
From dokumen.tips
(PPT) Electrochemical Reactions Redox reaction electrons transferred Electrochemical Cell Redox Reaction Redox reactions are a major class of chemical reactions in which there is an exchange of. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Electrochemical Cell Redox Reaction.
From slidetodoc.com
6 C Electrochemical Cells Redox reactions Redox reaction Electrochemical Cell Redox Reaction the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an. Electrochemical Cell Redox Reaction.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Electrochemical Cell Redox Reaction Redox reactions are a major class of chemical reactions in which there is an exchange of. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Electrochemical Cell Redox Reaction.
From byjus.com
In which direction is the flow of electrons in an indirect redox reaction? Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a voltaic cell (also known as a galvanic cell) is an electrochemical cell. Electrochemical Cell Redox Reaction.
From www.youtube.com
Balancing Redox Reactions in Acidic and Basic Conditions YouTube Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Redox reactions are a major class of chemical reactions in which there is an exchange of. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions.. Electrochemical Cell Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. if. Electrochemical Cell Redox Reaction.
From www.youtube.com
PREDICTING REDOX REACTIONS YouTube Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a voltaic cell (also known as a galvanic cell) is an electrochemical cell. Electrochemical Cell Redox Reaction.
From edenmeowbeard.blogspot.com
Redox Reaction Meaning Electrochemical Cell Redox Reaction a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. if a redox reaction can be split into half reactions it becomes possible to build a device, called. Electrochemical Cell Redox Reaction.
From www.researchgate.net
a) Electrochemical redox reaction mechanism of Na + ions with Na 4 C 8 Electrochemical Cell Redox Reaction Redox reactions are a major class of chemical reactions in which there is an exchange of. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to. Electrochemical Cell Redox Reaction.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Cell Redox Reaction Redox reactions are a major class of chemical reactions in which there is an exchange of. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. if a redox reaction can be split into half reactions it becomes possible to build a device, called an. Electrochemical Cell Redox Reaction.
From www.slideserve.com
PPT Introduction to electrochemical systems PowerPoint Presentation Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. Redox reactions are a major class of chemical reactions in which there is an exchange of. an. Electrochemical Cell Redox Reaction.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Electrochemical Cell Redox Reaction a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Redox reactions are a major class of chemical reactions in which there is an exchange of. . Electrochemical Cell Redox Reaction.
From wisc.pb.unizin.org
M18Q8 Electrolysis Chem 103/104 Resource Book Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. the redox reaction is faradic reaction, which is defined as reaction involved with. Electrochemical Cell Redox Reaction.
From www.biologyonline.com
Redox reaction Definition and Examples Biology Online Dictionary Electrochemical Cell Redox Reaction the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. Electrochemical Cell Redox Reaction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical. Electrochemical Cell Redox Reaction.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. Redox reactions are a major class of chemical reactions in which there is an exchange. Electrochemical Cell Redox Reaction.
From alevelchemistry.co.uk
Redox and Electrode Potential ALevel Chemistry Revision Notes Electrochemical Cell Redox Reaction Redox reactions are a major class of chemical reactions in which there is an exchange of. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate.. Electrochemical Cell Redox Reaction.
From saylordotorg.github.io
Applications of Redox Reactions Voltaic Cells Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Redox reactions are a major class of chemical reactions in which there is an. Electrochemical Cell Redox Reaction.
From www.slideserve.com
PPT Redox Reactions and Electrochemistry PowerPoint Presentation Electrochemical Cell Redox Reaction if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. Electrochemical Cell Redox Reaction.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Redox reactions are a major class of chemical reactions in which there is an exchange of. the redox reaction is faradic reaction, which is defined as reaction involved with electron transfer from/to electrode to/from ions. a. Electrochemical Cell Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. a voltaic cell (also known as a galvanic cell) is an electrochemical. Electrochemical Cell Redox Reaction.
From www.blitznotes.org
Redox Electrochemical Cell Redox Reaction if a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a voltaic cell (also known as a galvanic cell) is an electrochemical. Electrochemical Cell Redox Reaction.
From www.researchgate.net
Electrochemical redox reaction mechanisms of OEMs and their Electrochemical Cell Redox Reaction an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Redox reactions are a major class of chemical reactions in which there is an exchange of. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to. Electrochemical Cell Redox Reaction.
From www.youtube.com
Electrochemistry L1 Electrochemical Cell Galvanic Cell Redox Electrochemical Cell Redox Reaction devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that. Electrochemical Cell Redox Reaction.