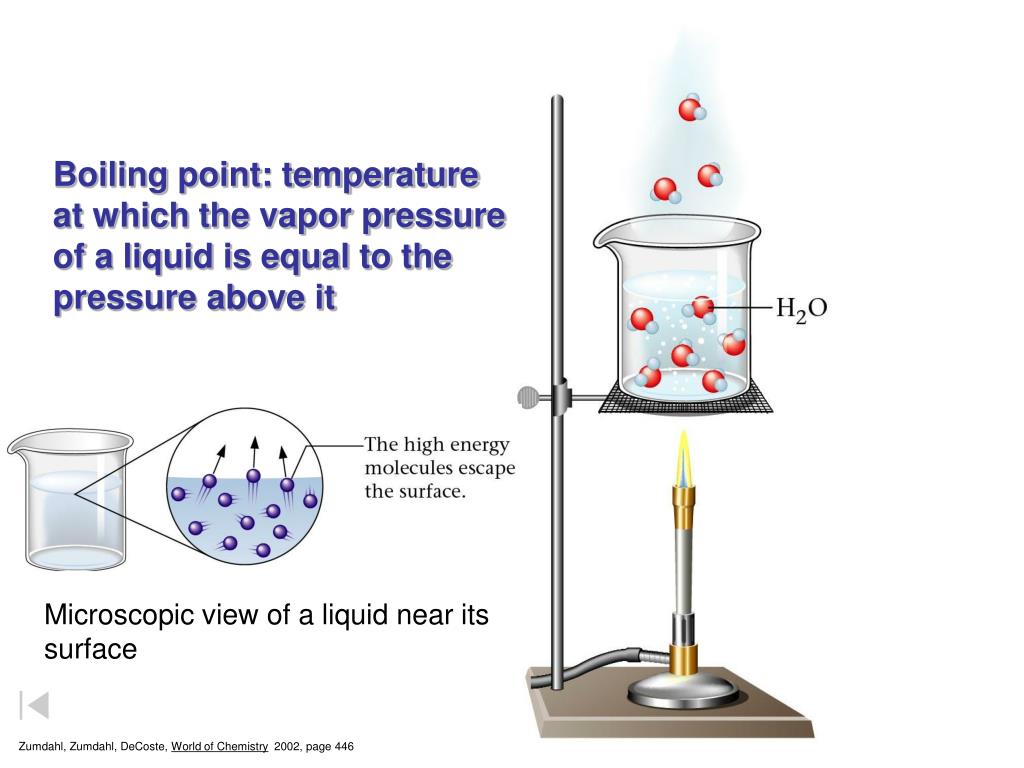
Boiling Point Of Liquid Pressure . The boiling point at different levels of pressure can be found out in. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. For example, at sea level, water boils at 212°f (100°c). The change from a liquid phase to. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point is the temperature at which a liquid substance changes to its gaseous phase. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This happens when the vapor. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. Temperature given as °c, °f, k and °r. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor.
from www.slideserve.com
For example, at sea level, water boils at 212°f (100°c). The boiling point is the temperature at which a liquid substance changes to its gaseous phase. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This happens when the vapor. The boiling point at different levels of pressure can be found out in. The change from a liquid phase to. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The boiling point is the temperature at which a liquid substance changes to its gaseous phase. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. The boiling point at different levels of pressure can be found out in. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The change from a liquid phase to. Temperature given as °c, °f, k and °r. For example, at sea level, water boils at 212°f (100°c). Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This happens when the vapor. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point at different levels of pressure can be found out in. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the. Boiling Point Of Liquid Pressure.
From morioh.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Boiling Point Of Liquid Pressure The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The change from a liquid phase. Boiling Point Of Liquid Pressure.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. Temperature given as °c, °f, k and °r. The boiling point at different levels of pressure can be found out in. Online calculator, figures and tables showing boiling points of water. Boiling Point Of Liquid Pressure.
From www.youtube.com
Vapor Pressure and Boiling YouTube Boiling Point Of Liquid Pressure The change from a liquid phase to. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point is the temperature at which a liquid substance changes to. Boiling Point Of Liquid Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Boiling Point Of Liquid Pressure The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. For example, at sea level, water boils at 212°f (100°c). Boiling occurs. Boiling Point Of Liquid Pressure.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of Liquid Pressure The boiling point at different levels of pressure can be found out in. Temperature given as °c, °f, k and °r. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). For example, at sea level, water boils at 212°f (100°c). Boiling occurs when the vapor pressure of. Boiling Point Of Liquid Pressure.
From www.engineeringtoolbox.com
Water Pressure and Boiling Points Boiling Point Of Liquid Pressure This happens when the vapor. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point is the temperature at which a liquid substance changes to its gaseous phase. The boiling point at different levels of pressure can be found out in. The change. Boiling Point Of Liquid Pressure.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Boiling Point Of Liquid Pressure The boiling point is the temperature at which a liquid substance changes to its gaseous phase. For example, at sea level, water boils at 212°f (100°c). Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). Temperature given as °c, °f, k and °r. The boiling point at. Boiling Point Of Liquid Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point is the temperature at which a liquid substance changes to its gaseous phase.. Boiling Point Of Liquid Pressure.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. The boiling point at different levels of pressure can be found out in. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. This. Boiling Point Of Liquid Pressure.
From www.youtube.com
Boiling point and external pressure YouTube Boiling Point Of Liquid Pressure The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. Boiling occurs when the vapor pressure of a liquid equals the air. Boiling Point Of Liquid Pressure.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and Boiling Point Of Liquid Pressure The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point is the temperature at which a liquid substance changes to its gaseous phase. The change from a liquid phase to. 95 rows when water is heated up under pressure, its boiling point is. Boiling Point Of Liquid Pressure.
From www.youtube.com
For a liquid, normal boiling point is `173^()C` . Then at 2 atm Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. Temperature given as °c, °f, k and °r. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. 95 rows when. Boiling Point Of Liquid Pressure.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Boiling Point Of Liquid Pressure The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The change from a liquid phase to. For example, at sea level, water boils at 212°f (100°c). Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling. Boiling Point Of Liquid Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. For example, at sea level, water boils at 212°f (100°c). Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. This happens when the vapor. The boiling point is defined as the temperature. Boiling Point Of Liquid Pressure.
From www.globalspec.com
Chapter 3 Physical Properties of Fluids Vapor Pressure and Boiling Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The change from a liquid phase to. Temperature given as °c, °f, k and °r. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above. Boiling Point Of Liquid Pressure.
From byjus.com
How does external pressure affect the boiling point Boiling Point Of Liquid Pressure The boiling point is the temperature at which a liquid substance changes to its gaseous phase. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid. Boiling Point Of Liquid Pressure.
From www.youtube.com
Calculate Boiling Point of Titanium at Different Pressure YouTube Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid. Boiling Point Of Liquid Pressure.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Boiling Point Of Liquid Pressure The change from a liquid phase to. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. For example, at sea level, water boils at 212°f (100°c). This happens when the vapor. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the. Boiling Point Of Liquid Pressure.
From www.pinterest.com
Atmospheric pressure and boiling point of water table Boiling point Boiling Point Of Liquid Pressure Temperature given as °c, °f, k and °r. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 95 rows when water is heated up under. Boiling Point Of Liquid Pressure.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). The boiling point at different levels of pressure can be found out in. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above. Boiling Point Of Liquid Pressure.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Boiling Point Of Liquid Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The boiling point is the temperature at which a liquid substance changes to its gaseous. Boiling Point Of Liquid Pressure.
From www.chegg.com
Solved The boiling point of a liquid is the temperature at Boiling Point Of Liquid Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. Temperature given as °c, °f, k and °r. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. For example, at sea level, water boils at 212°f (100°c).. Boiling Point Of Liquid Pressure.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). The boiling point is the temperature at which a liquid substance changes to its gaseous phase. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. The boiling point at. Boiling Point Of Liquid Pressure.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Boiling Point Of Liquid Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. This happens when the vapor. For example, at sea level, water boils at 212°f (100°c). The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The. Boiling Point Of Liquid Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Boiling Point Of Liquid Pressure The change from a liquid phase to. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). For example, at sea level, water boils at 212°f (100°c). The boiling point. Boiling Point Of Liquid Pressure.
From courses.lumenlearning.com
Phase Diagrams Chemistry Boiling Point Of Liquid Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the. Boiling Point Of Liquid Pressure.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Of Liquid Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The change from a liquid phase to. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point at different levels of pressure can be found. Boiling Point Of Liquid Pressure.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure Temperature given as °c, °f, k and °r. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). Boiling is the process by which. Boiling Point Of Liquid Pressure.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure The boiling point at different levels of pressure can be found out in. For example, at sea level, water boils at 212°f (100°c). Temperature given as °c, °f, k and °r. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid, and the liquid changes into a. Boiling Point Of Liquid Pressure.
From www.kentchemistry.com
Determine Boiling Point from Vapor Pressure Boiling Point Of Liquid Pressure Boiling occurs when the vapor pressure of a liquid equals the air pressure of the atmosphere above the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, at sea level, water boils at 212°f (100°c). Online calculator, figures and tables showing boiling points of water at. Boiling Point Of Liquid Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Boiling Point Of Liquid Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which a liquid substance changes to its gaseous phase. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling. Boiling Point Of Liquid Pressure.
From www.pinterest.com
Mastering Pressure Cooking Understanding Boiling Point at Different Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). Temperature given as °c, °f, k and °r. The boiling point at different levels of pressure can be found out in. The change from a liquid phase to. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding. Boiling Point Of Liquid Pressure.
From mavink.com
Water Boiling Point Vs Pressure Chart Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point is the temperature at which a liquid substance changes to its gaseous phase. The boiling point of a substance is the temperature at which the. Boiling Point Of Liquid Pressure.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Boiling Point Of Liquid Pressure For example, at sea level, water boils at 212°f (100°c). The boiling point at different levels of pressure can be found out in. This happens when the vapor. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Boiling occurs when the vapor pressure of a. Boiling Point Of Liquid Pressure.