Lyme Disease Vaccine Phase 3 . Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials.
from www.bbc.com
Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease.
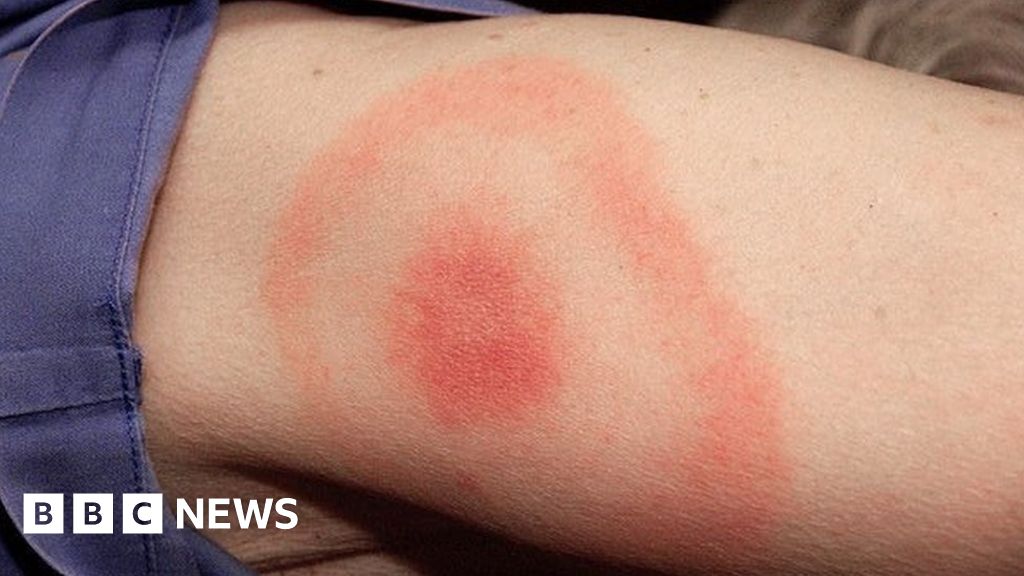
Lyme disease can be diagnosed by 'bull's eye' rash alone
Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials.
From healthsciences.arizona.edu
What it Takes to Create a Vaccine Health Sciences Connect Lyme Disease Vaccine Phase 3 Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Valneva and pfizer have developed a lyme disease vaccine candidate,. Lyme Disease Vaccine Phase 3.
From www.medicalnewstoday.com
Pfizer, Valneva launch phase 3 trials for Lyme disease vaccine Lyme Disease Vaccine Phase 3 Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and its. Lyme Disease Vaccine Phase 3.
From www.austinholisticdr.com
Symptoms and Stages of Lyme Disease Infinity Wellness Center Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials.. Lyme Disease Vaccine Phase 3.
From www.cnn.com
Only Lyme disease vaccine in development goes to Phase 3 trials CNN Lyme Disease Vaccine Phase 3 Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and. Lyme Disease Vaccine Phase 3.
From blogs.shu.edu
RNA Vaccines in Melanoma and Prostate Cancer Cancer Biology Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Vla15 is the most advanced. Lyme Disease Vaccine Phase 3.
From westchester.news12.com
Lyme disease vaccine heading into phase 3 trials Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a. Lyme Disease Vaccine Phase 3.
From www.genengnews.com
Lyme Disease Vaccine Uses mRNA to Encode Tick Saliva Proteins Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and valneva announced completion of recruitment for the phase 3. Lyme Disease Vaccine Phase 3.
From www.michiganmedicine.org
Lyme Disease Stages, Signs and Symptoms Everything You Need To Know Lyme Disease Vaccine Phase 3 Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and. Lyme Disease Vaccine Phase 3.
From newatlas.com
New Lyme disease vaccine targets decades of antivax misinformation Lyme Disease Vaccine Phase 3 Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Vla15 is the most advanced lyme disease vaccine candidate currently. Lyme Disease Vaccine Phase 3.
From www.skepticalraptor.com
Lyme disease vaccine update very good news from clinical trials Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced completion of. Lyme Disease Vaccine Phase 3.
From www.usatoday.com
VA recruiting volunteers for COVID19 vaccine Phase 3 clinical trials Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational. Lyme Disease Vaccine Phase 3.
From www.youtube.com
Vaccine for Lyme disease advances to phase 3 trial YouTube Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Valneva and. Lyme Disease Vaccine Phase 3.
From www.iflscience.com
A New Lyme Disease Vaccine Is Showing Promise IFLScience Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced that participants in their phase 3 valor trial. Lyme Disease Vaccine Phase 3.
From draxe.com
Lyme Disease Treatment Options, Causes, How to Prevent Dr. Axe Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a. Lyme Disease Vaccine Phase 3.
From www.nytimes.com
Developing New Guidelines on Lyme Disease The New York Times Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Valneva and pfizer. Lyme Disease Vaccine Phase 3.
From www.bbc.com
Lyme disease can be diagnosed by 'bull's eye' rash alone Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced that participants in their phase 3. Lyme Disease Vaccine Phase 3.
From www.nytimes.com
Lyme Disease Is Spreading Fast. Why Isn’t There a Vaccine? The New Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its. Lyme Disease Vaccine Phase 3.
From lymediseaseassociation.org
Lyme Vaccine Clinical Trials Reported as Positive for Pediatrics Lyme Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. This investigational. Lyme Disease Vaccine Phase 3.
From www.cnn.com
Only Lyme disease vaccine in development goes to Phase 3 trials CNN Lyme Disease Vaccine Phase 3 Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate.. Lyme Disease Vaccine Phase 3.
From lymediseaseassociation.org
Phase 3 VALOR Lyme Disease Trial Valneva and Pfizer Announce Primary Lyme Disease Vaccine Phase 3 Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease.. Lyme Disease Vaccine Phase 3.
From www.nytimes.com
Lyme Disease Is Spreading Fast. Why Isn’t There a Vaccine? The New Lyme Disease Vaccine Phase 3 Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Valneva and pfizer have developed a lyme disease. Lyme Disease Vaccine Phase 3.
From onlinepublichealth.gwu.edu
Producing Prevention How Vaccines Are Developed Online Public Health Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and. Lyme Disease Vaccine Phase 3.
From www.skepticalraptor.com
ValnevaPfizer Lyme disease vaccine — phase 3 trials to start soon Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase. Lyme Disease Vaccine Phase 3.
From www.cbc.ca
Major test of 1st potential Lyme disease vaccine in 20 years begins in Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Vla15 is the. Lyme Disease Vaccine Phase 3.
From lymediseaseassociation.org
New Lyme Vaccine Development from Tulane Lyme Disease Association Lyme Disease Vaccine Phase 3 Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease.. Lyme Disease Vaccine Phase 3.
From www.newscientist.com
Lyme disease vaccine found to be safe and effective in clinical trial Lyme Disease Vaccine Phase 3 Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study. Lyme Disease Vaccine Phase 3.
From lymediseaseassociation.org
Lyme Disease Vaccine Collaboration Announced Lyme Disease Association Lyme Disease Vaccine Phase 3 Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15. Lyme Disease Vaccine Phase 3.
From www.washingtonpost.com
As measles cases increase, a sharp call for vaccinations The Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15. Lyme Disease Vaccine Phase 3.
From insights.pfizer.com
Lyme Disease Vaccine Candidate Phase 3 Trial to Begin Pfizer Investor Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Pfizer and valneva announced that participants. Lyme Disease Vaccine Phase 3.
From www.caryinstitute.org
Lyme vaccine trial enrolling participants for phase three Lyme Disease Vaccine Phase 3 Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. Pfizer and valneva announced completion of recruitment for the phase 3 trial vaccine against lyme for outdoor recreationists (valor) for lyme disease vaccine candidate vla15. Pfizer and valneva. Lyme Disease Vaccine Phase 3.
From www.fiercebiotech.com
Pfizer, Valneva CRO problems to complete phase 3 recruitment Lyme Disease Vaccine Phase 3 Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme. Lyme Disease Vaccine Phase 3.
From www.usinenouvelle.com
Le vaccin Pfizer Valneva contre la maladie de Lyme entre en étude Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and its partner valneva se, a specialty vaccine company, announced the initiation of a phase 3 clinical study of their investigational lyme disease candidate. Valneva and pfizer. Lyme Disease Vaccine Phase 3.
From www.skepticalraptor.com
ValnevaPfizer Lyme disease vaccine — phase 3 trials to start soon Lyme Disease Vaccine Phase 3 Valneva and pfizer have developed a lyme disease vaccine candidate, vla15, that is currently in phase 3 human trials. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Pfizer and valneva announced completion. Lyme Disease Vaccine Phase 3.
From www.valleyvet.com
LymeVax Canine Lyme Disease Vaccine Zoetis Animal Health ( Vaccines Lyme Disease Vaccine Phase 3 This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. This investigational multivalent protein subunit vaccine uses an established mechanism of. Lyme Disease Vaccine Phase 3.
From seekingalpha.com
Pfizer and Valneva start phase 3 study of Lyme disease vaccine Lyme Disease Vaccine Phase 3 Pfizer and valneva announced that participants in their phase 3 valor trial for the lyme disease vaccine candidate vla15 have. Vla15 is the most advanced lyme disease vaccine candidate currently in clinical development. This investigational multivalent protein subunit vaccine uses an established mechanism of action for a lyme disease. This investigational multivalent protein subunit vaccine uses an established mechanism of. Lyme Disease Vaccine Phase 3.