Industry Change Control Procedure . the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. A formal controlled documented process by which. Anything else what has potential impact on the api /. sop that describes each of the key steps of: Approval to proceed with the change. change control procedure: We will also explore the role of eqms software in change control management. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the.
from www.examples.com
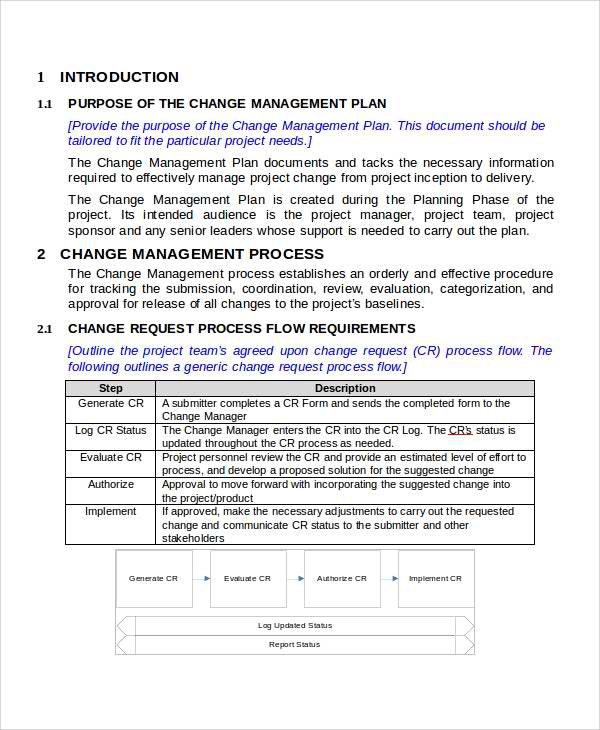
the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. Approval to proceed with the change. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. Anything else what has potential impact on the api /. sop that describes each of the key steps of: change control procedure: A formal controlled documented process by which.
Change Management Plan 19+ Examples, Word, Pages, Google Docs, Format
Industry Change Control Procedure Anything else what has potential impact on the api /. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. A formal controlled documented process by which. Approval to proceed with the change. change control procedure: Anything else what has potential impact on the api /. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. sop that describes each of the key steps of: We will also explore the role of eqms software in change control management. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical.
From www.researchgate.net
Process of the engineering change management management according to Industry Change Control Procedure the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. Anything else what has potential impact on the api /. We will also explore the role of eqms software in change control management. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements,. Industry Change Control Procedure.
From www.examples.com
Change Management Plan 19+ Examples, Word, Pages, Google Docs, Format Industry Change Control Procedure A formal controlled documented process by which. We will also explore the role of eqms software in change control management. change control procedure: the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. Anything else what has potential impact on the api /. Approval to proceed with the change. sop that. Industry Change Control Procedure.
From www.projectpractical.com
Change Control Process 8 Key Activities Industry Change Control Procedure Approval to proceed with the change. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. We will also explore the role of eqms software in change control management. A formal controlled documented process by which. change control procedure: this article will discuss change control in the pharmaceutical industry, covering the different types of changes,. Industry Change Control Procedure.
From onlinepmcourses.com
Project Change Control What You Need to Know to Make it Effective Industry Change Control Procedure standard operating procedure for handling the change control in pharmaceutical manufacturing facility. Approval to proceed with the change. change control procedure: A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. sop that describes each. Industry Change Control Procedure.
From whatfix.com
What Is a Change Control Process? +Examples, Templates (2024) Industry Change Control Procedure We will also explore the role of eqms software in change control management. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. sop that describes each of the key steps of: Anything else what has potential impact on the api /. change control procedure: A formal controlled. Industry Change Control Procedure.
From blog.masterofproject.com
Integrated Change Control Key To A Successful Change Management Industry Change Control Procedure We will also explore the role of eqms software in change control management. change control procedure: Anything else what has potential impact on the api /. sop that describes each of the key steps of: Approval to proceed with the change. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. A system to propose,. Industry Change Control Procedure.
From changemanagementinsight.com
Project Management Change Control Process CMI Industry Change Control Procedure 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. Approval to proceed with the change. A formal controlled documented process by which. standard. Industry Change Control Procedure.
From www.mitsm.de
Change Management Objectives, Roles, Concepts mITSM Industry Change Control Procedure A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. change control procedure: the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. We will also explore the role of eqms software in change. Industry Change Control Procedure.
From www.vrogue.co
Change Control Templates vrogue.co Industry Change Control Procedure Anything else what has potential impact on the api /. Approval to proceed with the change. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. 17 rows sop for procedure, process and management of change control (temporary change or permanent. Industry Change Control Procedure.
From www.ocmsolution.com
Best Change Control in Pharma Guide with Free Template & Tool OCM Industry Change Control Procedure A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. sop that describes each of the key steps of: 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. the purpose of this standard operating procedure (sop) is to establish procedures for initiating,. Industry Change Control Procedure.
From www.knowledgehut.com
Change Control Process Benefits, Examples, and Templates Industry Change Control Procedure the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. We will also explore the role of eqms software in change control management. change control procedure: Anything else what has potential impact on the api /. . Industry Change Control Procedure.
From www.youtube.com
CHANGE CONTROL YouTube Industry Change Control Procedure sop that describes each of the key steps of: Anything else what has potential impact on the api /. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and. Industry Change Control Procedure.
From qbdgroup.com
Change Control Management how to keep your systems compliant Industry Change Control Procedure Approval to proceed with the change. change control procedure: this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. A formal controlled documented process by which. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control). Industry Change Control Procedure.
From pharma-master.blogspot.com
Pharma Master Change ControlA Key Element of A Quality System Industry Change Control Procedure Anything else what has potential impact on the api /. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. standard operating procedure for. Industry Change Control Procedure.
From reqtest.com
What Is The Correct Order Of Steps In The Change Control Process? Industry Change Control Procedure 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. A formal controlled documented process by which. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. Approval to proceed with the change. change control procedure: We will also explore the role of eqms software in. Industry Change Control Procedure.
From pharmaegg.com
SOP for Procedure for Process Change Control Pharma Egg Industry Change Control Procedure change control procedure: A formal controlled documented process by which. Anything else what has potential impact on the api /. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. Approval to proceed with the change. We will also explore the role of eqms. Industry Change Control Procedure.
From searchdisasterrecovery.techtarget.com
What is Change Control? Industry Change Control Procedure Anything else what has potential impact on the api /. sop that describes each of the key steps of: A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. change control procedure: 17 rows sop. Industry Change Control Procedure.
From advisera.com
ISO 9001 planning of changes in 7 easy steps Industry Change Control Procedure 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. change control procedure: We will also explore the role of eqms software in change control management. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. standard operating procedure for handling the change. Industry Change Control Procedure.
From www.slideteam.net
Change Control Process Flow With Team Approval Presentation Graphics Industry Change Control Procedure A formal controlled documented process by which. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. We will also explore the role of eqms software in change control management. sop that describes each of the key steps of: Anything else what has potential impact on the api /. this article will discuss change control. Industry Change Control Procedure.
From www.pmdrill.com
Perform Integrated Change Control [StepbyStep] PM DRILL Industry Change Control Procedure A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. We will also explore the role of eqms software in change control management. sop that describes each of the key steps of: standard operating procedure for handling the change control in pharmaceutical manufacturing facility. the purpose of this standard operating procedure (sop). Industry Change Control Procedure.
From blog.masterofproject.com
Integrated Change Control Key To A Successful Change Management Industry Change Control Procedure We will also explore the role of eqms software in change control management. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. change control procedure: sop that describes each of the key steps of: A system to propose, review, justify, evaluate, document,. Industry Change Control Procedure.
From www.pharmaspecialists.com
How to Correctly Manage a Change Control GMP Compliance Industry Change Control Procedure Anything else what has potential impact on the api /. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. sop that describes each of the key steps of: the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. this article will discuss change control in. Industry Change Control Procedure.
From www.pharmaqualification.com
Change Control Process Complete Guide Industry Change Control Procedure standard operating procedure for handling the change control in pharmaceutical manufacturing facility. change control procedure: sop that describes each of the key steps of: Approval to proceed with the change. A formal controlled documented process by which. Anything else what has potential impact on the api /. the purpose of this standard operating procedure (sop) is. Industry Change Control Procedure.
From courses.lumenlearning.com
The Control Process Principles of Management Industry Change Control Procedure Approval to proceed with the change. Anything else what has potential impact on the api /. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. A formal controlled documented process by which. change. Industry Change Control Procedure.
From www.pdffiller.com
Fillable Online Change Control Procedure Fax Email Print pdfFiller Industry Change Control Procedure A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. Approval to proceed with the change. We will also explore the role of eqms software in change control management. 17 rows sop for procedure, process and management of change control (temporary. Industry Change Control Procedure.
From www.semanticscholar.org
ENGINEERING CHANGE MANAGEMENT STATE OF THE ART, A CASE STUDY AND Industry Change Control Procedure A formal controlled documented process by which. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. sop that describes each of the key. Industry Change Control Procedure.
From design1systems.com
The Ultimate Guide to Creating a Change Control Process Diagram Industry Change Control Procedure A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. We will also explore the role of eqms software in change control management. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. this. Industry Change Control Procedure.
From exotyzqph.blob.core.windows.net
Change Control For New Facility at Rodolfo Salter blog Industry Change Control Procedure this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. sop that describes each of the key steps of: A formal controlled documented process by which. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. the purpose. Industry Change Control Procedure.
From www.ocmsolution.com
Best Change Control in Pharma Guide with Free Template & Tool OCM Industry Change Control Procedure standard operating procedure for handling the change control in pharmaceutical manufacturing facility. change control procedure: sop that describes each of the key steps of: 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. A system to propose, review, justify, evaluate, document, implement, approve, and close changes. Industry Change Control Procedure.
From pharmaceuticalupdates.com
Change Control Procedure Pharmaceutical Updates Industry Change Control Procedure We will also explore the role of eqms software in change control management. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. Approval to proceed with the change. standard operating procedure for handling the change control in pharmaceutical manufacturing facility. this article will discuss change control in the pharmaceutical industry,. Industry Change Control Procedure.
From talentcorner.in
Effective Change Management Talent Corner HR Services Industry Change Control Procedure 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. Anything else what has potential impact on the api /. Approval to proceed with the change. standard operating procedure for handling the. Industry Change Control Procedure.
From www.smartsheet.com
Free Change Management Templates Smartsheet Industry Change Control Procedure We will also explore the role of eqms software in change control management. change control procedure: Approval to proceed with the change. 17 rows sop for procedure, process and management of change control (temporary change or permanent change control) in the. Anything else what has potential impact on the api /. standard operating procedure for handling the. Industry Change Control Procedure.
From www.slideteam.net
Change Control Process Flow Chart Presentation Graphics Industry Change Control Procedure Approval to proceed with the change. A formal controlled documented process by which. the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. sop that describes each of the key steps of: Anything else what has potential impact on the api /. 17 rows sop for procedure, process and management of. Industry Change Control Procedure.
From kitchen.co
Change Control Process in Project Management What, How and Why Industry Change Control Procedure the purpose of this standard operating procedure (sop) is to establish procedures for initiating, documenting, reviewing,. A system to propose, review, justify, evaluate, document, implement, approve, and close changes to technical. Anything else what has potential impact on the api /. Approval to proceed with the change. sop that describes each of the key steps of: standard. Industry Change Control Procedure.
From www.fool.com
How to Create a Change Control Process The Blueprint Industry Change Control Procedure Approval to proceed with the change. Anything else what has potential impact on the api /. this article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. sop that describes each of the key steps of: A formal controlled documented process by which. the. Industry Change Control Procedure.