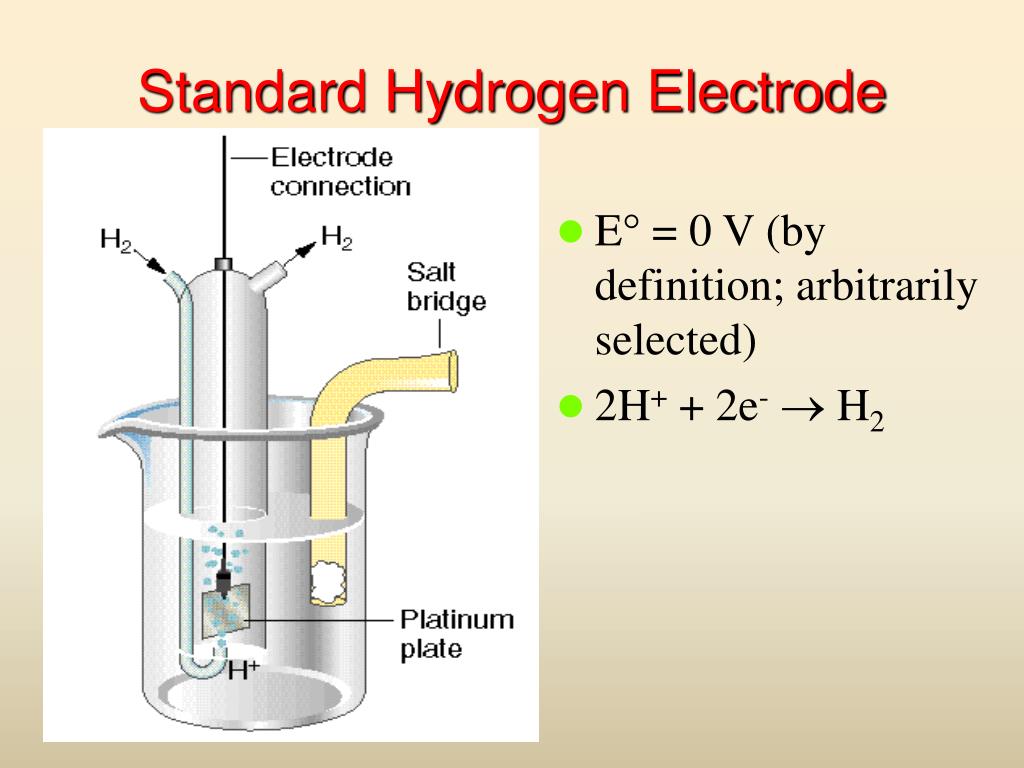
Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential . The structure of the standard hydrogen electrode is described. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). Examples of using the standard hydrogen electrode to determine reduction potentials are given. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a.
from www.slideserve.com
the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard hydrogen electrode is described.
PPT CHEM 160 General Chemistry II Lecture Presentation
Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. Examples of using the standard hydrogen electrode to determine reduction potentials are given. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she).
From slideplayer.com
Electrochemistry. ppt download Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. if the standard hydrogen electrode (she). Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). Examples of using the standard hydrogen electrode to determine reduction potentials are given. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. graphical representation of the onset potentials. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From mungfali.com
Electrode Potential Series Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the absolute. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the potential of the standard hydrogen electrode. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). the standard hydrogen electrode (she) is universally used for this purpose and is assigned. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential Examples of using the standard hydrogen electrode to determine reduction potentials are given. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the potential of. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). The structure of the standard hydrogen electrode is described. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. electrochemical potentials are conventionally referred to that of the standard. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the absolute. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential Examples of using the standard hydrogen electrode to determine reduction potentials are given. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. electrochemical potentials. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is described. if the standard hydrogen electrode (she) is set arbitrarily to have a potential. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. if the standard hydrogen electrode (she) is set. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From slideplayer.com
Electrochemistry. ppt download Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.youtube.com
Standard Hydrogen Electrode Definition ,Construction ,Working Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). The structure of the standard hydrogen electrode is described. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. graphical representation of the onset potentials of the her/hor and oer/orr measured using. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From slideplayer.com
Voltammetry and Polarography ppt download Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From slidetodoc.com
Standard Reference Electrode Standard Hydrogen Electrode SHE SHE Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Examples of using the standard hydrogen electrode to determine reduction potentials are given. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the absolute potential of the standard hydrogen electrode, she, was calculated. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the potential of the standard hydrogen electrode (she) is defined as 0 v under. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. the potential of the standard hydrogen electrode (she) is. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). if the standard hydrogen electrode (she) is set arbitrarily to have a potential. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). The structure of the standard hydrogen electrode is described. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From solvedlib.com
3 Draw a labeled diagram of standard hydrogen electro… SolvedLib Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential if the standard hydrogen electrode (she) is set arbitrarily to have a potential that is equal to 0 v, and has an. Examples of using the standard hydrogen electrode to determine reduction potentials are given. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). the absolute potential of the standard hydrogen electrode, she,. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential The structure of the standard hydrogen electrode is described. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. if the standard hydrogen electrode (she) is set arbitrarily to have a potential. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From schoolbag.info
4.2 Electrochemical Reactions Solutions of Electrolytes Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). Examples of using the standard hydrogen electrode to determine reduction potentials are given. graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. if the standard hydrogen electrode (she) is set arbitrarily to have a. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From slideplayer.com
Unit 09 Oxidation and Reduction ppt download Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). Examples of. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a thermodynamic cycle involving h 2 (g). The structure of the standard hydrogen electrode is described. electrochemical potentials are conventionally referred to that of the standard hydrogen electrode (she). graphical representation of the onset potentials of the her/hor and oer/orr measured using. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard hydrogen electrode is described. if the standard hydrogen electrode (she) is set arbitrarily to have a. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential. Standard Hydrogen Electrode Has An Arbitrarily Fixed Potential.