Chlorine Formal Charge . A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. To calculate the formal charge of an atom, we start by: Formal charge and free radicals. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. Compute formal charges for atoms in any lewis structure. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Explain the concept of resonance and draw. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Use formal charges to identify the most reasonable lewis structure for a given. How to calculate formal charge. Free radicals are very reactive and there are very few stable free. Explain the formal charge of chlorine in the cl 2 lewis structure. Use formal charges to identify the most reasonable lewis structure for a given molecule. A free radical is a molecule that has one electron.
from carakruwlambert.blogspot.com
To calculate the formal charge of an atom, we start by: Use formal charges to identify the most reasonable lewis structure for a given molecule. Free radicals are very reactive and there are very few stable free. How to calculate formal charge. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. Use formal charges to identify the most reasonable lewis structure for a given molecule. A free radical is a molecule that has one electron. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Use formal charges to identify the most reasonable lewis structure for a given. Formal charge and free radicals.
Calculate the Formal Charge on the Chlorine Cl Atom CarakruwLambert
Chlorine Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Use formal charges to identify the most reasonable lewis structure for a given molecule. How to calculate formal charge. Compute formal charges for atoms in any lewis structure. Free radicals are very reactive and there are very few stable free. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). A free radical is a molecule that has one electron. Explain the concept of resonance and draw. Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given. Use formal charges to identify the most reasonable lewis structure for a given molecule. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Explain the formal charge of chlorine in the cl 2 lewis structure. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. Formal charge and free radicals. To calculate the formal charge of an atom, we start by:
From www.numerade.com
SOLVED Short answer is enough. Draw the best Lewis structure for ClO⁻ and determine the formal Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given. Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the formal charge of chlorine in the cl 2 lewis structure. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). A free. Chlorine Formal Charge.
From byjus.com
Calculate the formal charge on Cl atom in HCIO4. Chlorine Formal Charge Explain the concept of resonance and draw. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the formal charge of chlorine in the cl 2. Chlorine Formal Charge.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Chlorine Formal Charge Explain the formal charge of chlorine in the cl 2 lewis structure. Compute formal charges for atoms in any lewis structure. Formal charge and free radicals. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. Explain the concept of resonance and draw. A free radical is a molecule. Chlorine Formal Charge.
From www.numerade.com
SOLVED Draw the best Lewis structure for CIO and determine the formal charge on chlorine B Chlorine Formal Charge Explain the concept of resonance and draw. Explain the concept of resonance and draw. A free radical is a molecule that has one electron. Free radicals are very reactive and there are very few stable free. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Formal charge and free radicals. Use formal charges to identify the most. Chlorine Formal Charge.
From www.chegg.com
Solved SA) What is the formal charge on the chlorine atom in Chlorine Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Explain the concept of resonance and draw. How to calculate formal charge. A free radical is a molecule that has one electron. Explain the concept of resonance and draw. Compute formal charges for atoms in any lewis structure. Explain the formal charge of chlorine in the cl 2. Chlorine Formal Charge.
From www.coursehero.com
The chlorine containing oxyacid that has Cl in an oxidation state oxidation Course Hero Chlorine Formal Charge Compute formal charges for atoms in any lewis structure. Explain the formal charge of chlorine in the cl 2 lewis structure. To calculate the formal charge of an atom, we start by: Formal charge and free radicals. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared. Chlorine Formal Charge.
From www.chegg.com
Solved What is the Formal Charge of the Chlorine atom in the Chlorine Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). To calculate the formal charge of an atom, we start by: Formal charge and free radicals. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. How to calculate formal charge. A formal charge (fc) is the. Chlorine Formal Charge.
From www.slideserve.com
PPT VSEPR PowerPoint Presentation, free download ID2420118 Chlorine Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Explain the formal charge of chlorine in the cl 2 lewis structure. To calculate the formal charge of an atom, we start by: Compute formal charges for atoms in any lewis structure. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that. Chlorine Formal Charge.
From www.youtube.com
What is the formal charge of chlorine in the molecules Cl2, BeCl2, and ClF5? YouTube Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given. Use formal charges to identify the most reasonable lewis structure for a given molecule. Compute formal charges for atoms in any lewis structure. To calculate the formal charge of an atom, we start by: Free radicals are very reactive and there are very few stable free. How. Chlorine Formal Charge.
From www.chegg.com
Solved What is the formal charge of the Chlorine and Chlorine Formal Charge Compute formal charges for atoms in any lewis structure. Explain the formal charge of chlorine in the cl 2 lewis structure. How to calculate formal charge. Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given. A formal charge (fc) is the charge assigned to an atom in a molecule,. Chlorine Formal Charge.
From h-o-m-e.org
The Formal Charge in Chlorine's Most Common Form Perchlorate Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). A free radical is a molecule that has one electron. Explain the concept of resonance and draw. Free radicals are very reactive and there are very few stable free. To calculate the formal charge of. Chlorine Formal Charge.
From www.coursehero.com
[Solved] Calculate the formal charge on the chlorine in chlorate Course Hero Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given molecule. How to calculate formal charge. Use formal charges to identify the most reasonable lewis structure for a given. A free radical is a molecule that has one electron. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Compute formal charges for atoms in. Chlorine Formal Charge.
From www.youtube.com
ClF5 Lewis Structure How to Draw the Lewis Structure for ClF5 (Chlorine Pentafluoride) YouTube Chlorine Formal Charge Explain the concept of resonance and draw. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. How to calculate formal charge. Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given. Compute formal charges for atoms in any lewis structure.. Chlorine Formal Charge.
From www.chegg.com
Solved What is the formal charge of the chlorine atom in Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). A free radical is a molecule that has one electron. A formal charge (fc) is. Chlorine Formal Charge.
From byjus.com
What is the Formal charge on Cl in HClO4? Chlorine Formal Charge To calculate the formal charge of an atom, we start by: Explain the concept of resonance and draw. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given molecule. A formal. Chlorine Formal Charge.
From www.youtube.com
What is the formal charge on the chlorine atom in the oxyacid HOClO_2 if it contains single Chlorine Formal Charge Explain the concept of resonance and draw. A free radical is a molecule that has one electron. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. Explain the. Chlorine Formal Charge.
From www.numerade.com
SOLVED 'Cl Q' For the Lewis diagram, above, determine The formal charge on the chlorine atom Chlorine Formal Charge Explain the concept of resonance and draw. Explain the concept of resonance and draw. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). To calculate the formal charge of an atom, we start by: Evaluating the number of valence electrons (ve) the neutral atom has (e.g. A formal charge (fc) is the charge assigned to an atom. Chlorine Formal Charge.
From www.numerade.com
SOLVED (a) Determine the formal charge on the chlorine atom in the hypochlorite ion, ClO, and Chlorine Formal Charge Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given molecule. A free radical is a molecule that has one electron. Formal charge and free radicals. 3 for boron, 4 for carbon, 5 for nitrogen, and. Chlorine Formal Charge.
From www.chegg.com
Solved What is the formal charge of the chlorine atom in the Chlorine Formal Charge Explain the concept of resonance and draw. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. How to calculate formal charge. Compute formal charges for atoms in any lewis structure. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain. Chlorine Formal Charge.
From www.numerade.com
SOLVED a. Determine the formal charge on the chlorine atom in the hypochlorite ion ClO and the Chlorine Formal Charge Free radicals are very reactive and there are very few stable free. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Compute formal charges for atoms in any lewis structure. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless. Chlorine Formal Charge.
From www.chegg.com
Solved 3. What is the formal charge on the chlorine atom in Chlorine Formal Charge Formal charge and free radicals. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Explain the formal charge of chlorine in the cl 2 lewis structure. Free radicals. Chlorine Formal Charge.
From www.chegg.com
Solved In the following Lewis structure for ClO3F, chlorine Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given. To calculate the formal charge of an atom, we start by: Formal charge and free radicals. How to calculate formal charge. Explain the formal charge of chlorine in the cl 2 lewis structure. Explain the concept of resonance and draw. 3 for boron, 4 for carbon, 5. Chlorine Formal Charge.
From h-o-m-e.org
The Formal Charge in Chlorine's Most Common Form Perchlorate Chlorine Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Free radicals are very reactive and there are very few stable free. Use formal charges to identify the most reasonable lewis structure for a given molecule. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Explain the formal charge of chlorine in the cl 2. Chlorine Formal Charge.
From www.numerade.com
SOLVED Consider the first Lewis structure for NOCl The formal charge on the oxygen atom is Chlorine Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. How to calculate formal charge. Use formal charges to identify the most reasonable lewis structure for a given. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Compute. Chlorine Formal Charge.
From www.chegg.com
Solved The formal charge on the chlorine atom in ClO3−drawn Chlorine Formal Charge How to calculate formal charge. Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the formal charge of chlorine in the cl 2 lewis structure. A free radical is a molecule that has one electron. Explain the concept of resonance and draw. 3 for boron, 4 for. Chlorine Formal Charge.
From carakruwlambert.blogspot.com
Calculate the Formal Charge on the Chlorine Cl Atom CarakruwLambert Chlorine Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless. Chlorine Formal Charge.
From www.chegg.com
Solved 2. What is the formal charge on the chlorine atom in Chlorine Formal Charge Evaluating the number of valence electrons (ve) the neutral atom has (e.g. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). How to calculate formal charge. Compute formal charges for atoms in any lewis structure. To calculate the formal charge of an atom, we start by: A formal charge (fc) is the charge assigned to an atom. Chlorine Formal Charge.
From www.chegg.com
Solved QUESTION 9 The formal charge on Chlorine in the Chlorine Formal Charge A free radical is a molecule that has one electron. Compute formal charges for atoms in any lewis structure. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Formal charge and free radicals. Use formal charges to identify the most reasonable lewis structure for a given. A formal charge (fc) is the charge assigned to an atom. Chlorine Formal Charge.
From www.chegg.com
Solved Calculate the formal charge for Chlorine. Then Chlorine Formal Charge A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative. Use formal charges to identify the most reasonable lewis structure for a given. Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Use formal charges to identify the. Chlorine Formal Charge.
From www.youtube.com
How to Calculate the Formal Charges for HClO4 (Perchloric acid) YouTube Chlorine Formal Charge Compute formal charges for atoms in any lewis structure. Formal charge and free radicals. Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given. Explain the concept of resonance and draw. Free radicals are very reactive and there are very few stable free.. Chlorine Formal Charge.
From www.coursehero.com
[Solved] Draw the Lewis structure of chlorine pentafluoride. Identify BOTH... Course Hero Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given. Use formal charges to identify the most reasonable lewis structure for a given molecule. Explain the concept of resonance and draw. A free radical is a molecule that has one electron. Compute formal charges for atoms in any lewis structure. How to calculate formal charge. Formal charge. Chlorine Formal Charge.
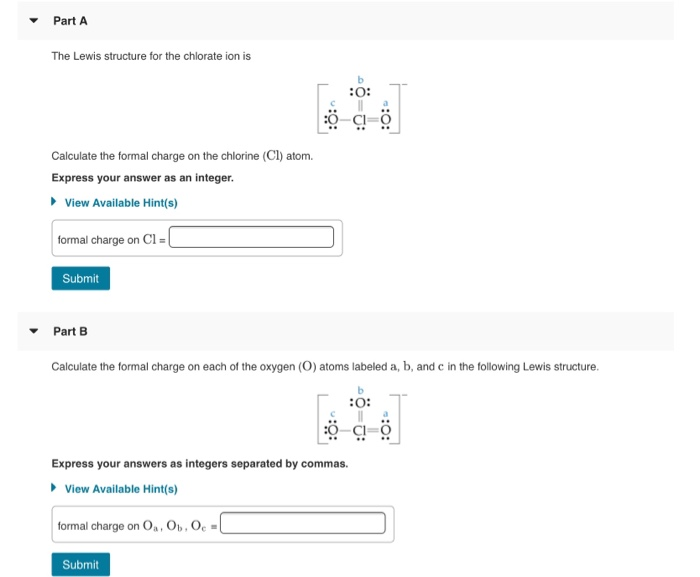
From www.chegg.com
Solved Part A Calculate the formal charge on the chlorine Chlorine Formal Charge 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given. Free radicals are very reactive and there are very few stable free. Explain the concept of resonance and draw. A formal. Chlorine Formal Charge.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge Pharmacy Resource Book Chlorine Formal Charge To calculate the formal charge of an atom, we start by: Evaluating the number of valence electrons (ve) the neutral atom has (e.g. Use formal charges to identify the most reasonable lewis structure for a given molecule. Formal charge and free radicals. 3 for boron, 4 for carbon, 5 for nitrogen, and so on). Explain the concept of resonance and. Chlorine Formal Charge.
From ezra-kwells.blogspot.com
Calculate the Formal Charge on the Chlorine Cl Atom Chlorine Formal Charge Use formal charges to identify the most reasonable lewis structure for a given molecule. Use formal charges to identify the most reasonable lewis structure for a given. Free radicals are very reactive and there are very few stable free. Formal charge and free radicals. A free radical is a molecule that has one electron. To calculate the formal charge of. Chlorine Formal Charge.
From www.chegg.com
Solved The formal charge of the chlorine 14 central atom in Chlorine Formal Charge Free radicals are very reactive and there are very few stable free. Compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure for a given. Formal charge and free radicals. Explain the concept of resonance and draw. Use formal charges to identify the most reasonable lewis structure for a given molecule.. Chlorine Formal Charge.