Redox Titration Permanganate And Iron . To determine the mass of fe2+ in an iron. This is a full demonstration of a redox titration, showing how to make a standard solution. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. What is the molarity of the.
from www.chegg.com
To determine the mass of fe2+ in an iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. This is a full demonstration of a redox titration, showing how to make a standard solution. What is the molarity of the. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium.
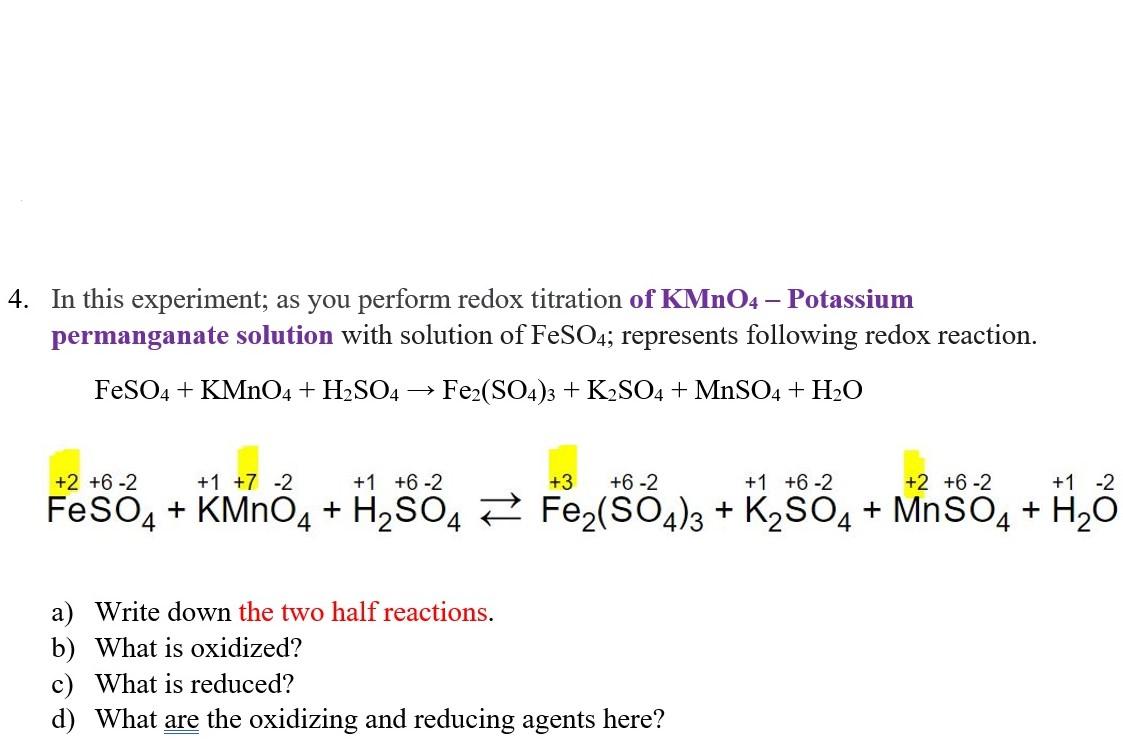
Solved 4. In this experiment; as you perform redox titration
Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. This is a full demonstration of a redox titration, showing how to make a standard solution. To determine the mass of fe2+ in an iron. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. What is the molarity of the. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999).
From www.youtube.com
Redox titration between iron and permanganate YouTube Redox Titration Permanganate And Iron The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the mass of fe2+ in an iron. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. To avoid the problem we can add mn 2+ to the solution to lower redox potential. Redox Titration Permanganate And Iron.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Redox Titration Permanganate And Iron What is the molarity of the. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. To avoid the problem we can add mn 2+ to the solution to lower redox potential. Redox Titration Permanganate And Iron.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Redox Titration Permanganate And Iron This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. This is a full demonstration of a redox titration, showing how to make a standard solution. To determine the mass of fe2+ in an iron. What is the molarity of the. In this titration reaction, the fe2+. Redox Titration Permanganate And Iron.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration Permanganate And Iron This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. What is. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Redox Titration Permanganate And Iron In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. Iron (ii) ions are titrated with potassium permanganate (kmno. Redox Titration Permanganate And Iron.
From www.numerade.com
SOLVED The concentration of iron(II) ions in water can be determined Redox Titration Permanganate And Iron To determine the mass of fe2+ in an iron. What is the molarity of the. This is a full demonstration of a redox titration, showing how to make a standard solution. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved Advance Study Assignment Determination of Iron by Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. In this titration reaction, the fe2+ ions react with permanganate ions,. Redox Titration Permanganate And Iron.
From www.alamy.com
Potassium permanganate titration. At the start of a redox titration Redox Titration Permanganate And Iron To determine the mass of fe2+ in an iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. This is a full demonstration of a redox titration, showing how to make a standard. Redox Titration Permanganate And Iron.
From www.science-revision.co.uk
Further redox reactions Redox Titration Permanganate And Iron Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). What is the molarity of the. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as. Redox Titration Permanganate And Iron.
From www.numerade.com
SOLVED Iron supplement tablets contain iron (II) ions. The amount of Redox Titration Permanganate And Iron To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. To determine the mass of fe2+ in an iron. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. It. Redox Titration Permanganate And Iron.
From phonemadness1.blogspot.com
Read Potassium Permanganate And Iron Sulfate Equation Updated Phone Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). The redox titration of permanganate ions is commonly used to determine the iron content of a. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved Advance Study Assignment Determination of Iron by Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. This method involves reacting the iron in the tablet with a. Redox Titration Permanganate And Iron.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Redox Titration Permanganate And Iron To determine the mass of fe2+ in an iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To avoid the problem we. Redox Titration Permanganate And Iron.
From www.academia.edu
(PDF) Determination of iron by redox titration Farhang Awlqadr Redox Titration Permanganate And Iron Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Iron tablet titration is a common laboratory method used to. Redox Titration Permanganate And Iron.
From www.slideserve.com
PPT Booklet to help with Unit 27 PowerPoint Presentation ID1951670 Redox Titration Permanganate And Iron Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. What is the molarity of the. To determine the mass of fe2+ in an iron. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. To avoid the problem we. Redox Titration Permanganate And Iron.
From www.numerade.com
SOLVEDIn the redox titration of iron(Il) with permanganate; the orange Redox Titration Permanganate And Iron Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. What is the molarity of the. The redox titration of. Redox Titration Permanganate And Iron.
From rileighrobertson2017.weebly.com
Permanganate Titration Rileigh Robertson Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. What is the molarity of the. To determine the mass of fe2+ in an iron. It takes 38.29 ml of a. Redox Titration Permanganate And Iron.
From www.youtube.com
Redox Titration (Iron) YouTube Redox Titration Permanganate And Iron The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such. Redox Titration Permanganate And Iron.
From www.youtube.com
Redox Titration between MnO4 and Fe2+ YouTube Redox Titration Permanganate And Iron This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. This is a full demonstration of a redox titration, showing how to make a standard solution. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). The redox. Redox Titration Permanganate And Iron.
From www.youtube.com
REDOX TITRATION IRON 2+ WITH POTASSIUM PERMANGANATE YouTube Redox Titration Permanganate And Iron To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. This is a full demonstration of a redox titration, showing how to make a standard solution. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved Date 3/31/20 Determination of Iron by Redox Redox Titration Permanganate And Iron In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. This is a full demonstration of a redox titration, showing how to make a standard solution. What is the molarity of the. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron. Redox Titration Permanganate And Iron.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Redox Titration Permanganate And Iron Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. To determine the mass of fe2+ in an. Redox Titration Permanganate And Iron.
From www.slideshare.net
Potassium permanganate titrations Redox Titration Permanganate And Iron The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the mass of fe2+ in an iron. This is a full demonstration of a redox titration, showing how to make a standard solution. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary. Redox Titration Permanganate And Iron.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration Permanganate And Iron To determine the mass of fe2+ in an iron. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to produce a purple colour. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. What is the. Redox Titration Permanganate And Iron.
From www.numerade.com
SOLVED Determination of Iron by Reaction with Prelaboratory Assignment Redox Titration Permanganate And Iron The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. What is the molarity of the. To determine the mass of fe2+ in an iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement. Redox Titration Permanganate And Iron.
From studylib.net
Determination of Iron by Redox Titration Redox Titration Permanganate And Iron What is the molarity of the. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the mass of fe2+ in an iron. In this titration reaction, the. Redox Titration Permanganate And Iron.
From cityraven.com
🎉 Standardisation of kmno4. Permanganate Titrations. 20190117 Redox Titration Permanganate And Iron To determine the mass of fe2+ in an iron. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method involves reacting the. Redox Titration Permanganate And Iron.
From www.youtube.com
Redox titration lab permanganate and iron (II) under acidic Redox Titration Permanganate And Iron To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. What is the molarity of the. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. This method involves reacting the iron in the tablet with a solution of potassium permanganate (kmno 4 ) to. Redox Titration Permanganate And Iron.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Redox Titration Permanganate And Iron It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). What is the molarity of the. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. This is a full demonstration of a redox titration, showing how to make a standard solution. To avoid the problem we can add mn. Redox Titration Permanganate And Iron.
From studylib.net
Redox Titration Potassium Permanganate and Sodium Oxalate Redox Titration Permanganate And Iron It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this titration reaction, the fe2+. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved Experiment 9 Determination of Iron by Redox Redox Titration Permanganate And Iron Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement tablet. It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). The redox titration of permanganate ions is commonly used to determine the iron content of a. Redox Titration Permanganate And Iron.
From www.linstitute.net
CIE A Level Chemistry复习笔记6.3.3 Redox Systems翰林国际教育 Redox Titration Permanganate And Iron It takes 38.29 ml of a permanganate solution to titrate 0.2587 g of preimary standard nac2o4 (m = 133.999). The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to. Redox Titration Permanganate And Iron.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Redox Titration Permanganate And Iron To avoid the problem we can add mn 2+ to the solution to lower redox potential of the permanganate to the point where chloride. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. This is a full demonstration of a redox titration, showing how to make a standard solution. Potassium permanganate is the oxidizing. Redox Titration Permanganate And Iron.
From www.chegg.com
Solved Experiment 6 Advance Study Assignment Determination Redox Titration Permanganate And Iron What is the molarity of the. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. This is a full demonstration of a redox titration, showing how to make a standard solution. Iron tablet titration is a common laboratory method used to determine the amount of iron present in a sample, such as an iron supplement. Redox Titration Permanganate And Iron.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记8.2.1 Redox Titration翰林国际教育 Redox Titration Permanganate And Iron The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Potassium permanganate is the oxidizing agent, oxidizing iron (ii) to iron. In this titration reaction, the fe2+ ions react with permanganate ions, mno4− and acid h+. To avoid the problem we can add mn 2+ to the solution to. Redox Titration Permanganate And Iron.