What Is The Total Number Of Orbitals Associated With N=3 . The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. An s subshell only has one orbital. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. A p subshell has three. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. To calculate the maximum number of electrons in each energy. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution.
from courses.lumenlearning.com
The third electron shell has 3 subshells, which are 3s, 3p, and 3d. A p subshell has three. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. An s subshell only has one orbital. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +.
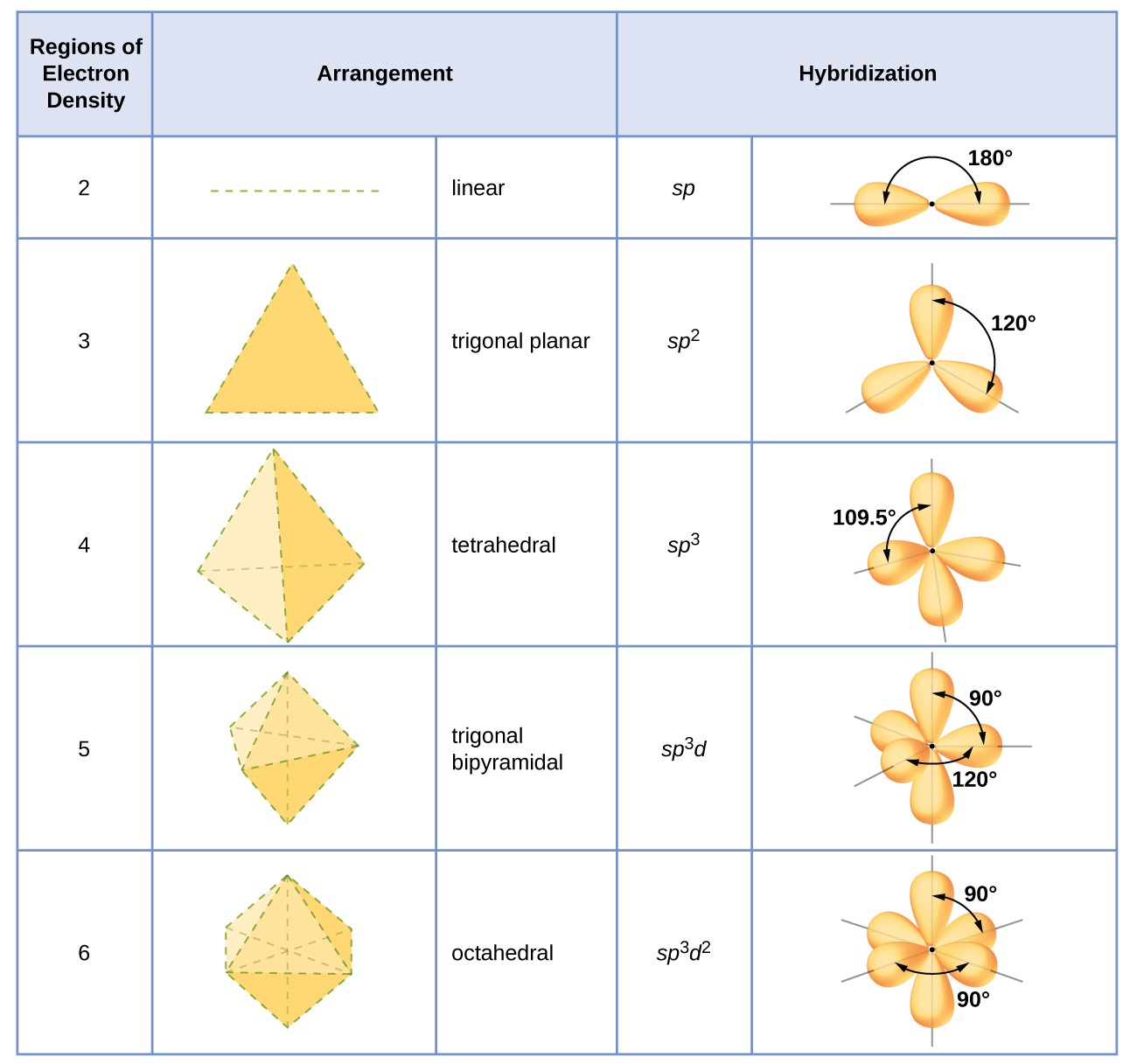
Hybrid Atomic Orbitals Chemistry for Majors
What Is The Total Number Of Orbitals Associated With N=3 \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. To calculate the maximum number of electrons in each energy. An s subshell only has one orbital. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The third electron shell has 3 subshells, which are 3s, 3p, and 3d. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. A p subshell has three. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and.
From www.youtube.com
What is the total number of orbitals associated with the principal quantum number n = 3 ? YouTube What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The third electron shell has 3 subshells, which are 3s, 3p, and 3d. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0. What Is The Total Number Of Orbitals Associated With N=3.
From www.chegg.com
Solved 4) What is the total number of orbitals associated What Is The Total Number Of Orbitals Associated With N=3 Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. The total number of orbitals in the n = 4. What Is The Total Number Of Orbitals Associated With N=3.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition, Detailed Explanation What Is The Total Number Of Orbitals Associated With N=3 \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. A p subshell has three. The. What Is The Total Number Of Orbitals Associated With N=3.
From www.chegg.com
Solved 4) What is the total number of orbitals associated What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The third electron shell has 3 subshells, which are 3s, 3p, and 3d. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin. What Is The Total Number Of Orbitals Associated With N=3.
From hubpages.com
Atoms and Atomic Structure hubpages What Is The Total Number Of Orbitals Associated With N=3 The third electron shell has 3 subshells, which are 3s, 3p, and 3d. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. A p subshell has three. For n = 3 there. What Is The Total Number Of Orbitals Associated With N=3.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is The Total Number Of Orbitals Associated With N=3 \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25. What Is The Total Number Of Orbitals Associated With N=3.
From slideplayer.com
Quantum Mechanics College Chemistry. ppt download What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. A p subshell has three. For n = 3 there are nine orbitals, for n = 4 there are 16. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
20. What is the total number of orbitals associated with the principal quantum number n=3 ? 1 What Is The Total Number Of Orbitals Associated With N=3 Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: A p subshell has three. The total number of orbitals possible for a given. What Is The Total Number Of Orbitals Associated With N=3.
From www.numerade.com
SOLVED What is the total number of orbitals associated with the principal quantum number n = 3? What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: An s subshell only has one orbital. For n = 3 there. What Is The Total Number Of Orbitals Associated With N=3.
From www.chegg.com
Solved what is the total number of orbitals associated with What Is The Total Number Of Orbitals Associated With N=3 To calculate the maximum number of electrons in each energy. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals,. What Is The Total Number Of Orbitals Associated With N=3.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is The Total Number Of Orbitals Associated With N=3 Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. The total number of orbitals in the n = 4. What Is The Total Number Of Orbitals Associated With N=3.
From www.slideshare.net
Orbitals electronconfiguration What Is The Total Number Of Orbitals Associated With N=3 An s subshell only has one orbital. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. Thus, we find a total of 16 orbitals in the. What Is The Total Number Of Orbitals Associated With N=3.
From byjus.com
What is the total number of orbitalsassociated with the principal quantum number n 3? What Is The Total Number Of Orbitals Associated With N=3 A p subshell has three. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. To calculate the maximum number of electrons in each energy. Thus, we. What Is The Total Number Of Orbitals Associated With N=3.
From courses.lumenlearning.com
Hybrid Atomic Orbitals Chemistry for Majors What Is The Total Number Of Orbitals Associated With N=3 The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom.. What Is The Total Number Of Orbitals Associated With N=3.
From www.youtube.com
What is the total number of orbitals associated with the principal quantum number n=3? YouTube What Is The Total Number Of Orbitals Associated With N=3 A p subshell has three. To calculate the maximum number of electrons in each energy. Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. The third electron shell has 3 subshells, which. What Is The Total Number Of Orbitals Associated With N=3.
From www.doubtnut.com
The total number of orbitals associated with the principal quantum num What Is The Total Number Of Orbitals Associated With N=3 \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2:. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
The total number of orbitals associated with the principal quantum number 5 is What Is The Total Number Of Orbitals Associated With N=3 For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. A p subshell has three. The number of orbitals for a given principal quantum number n can be. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
SUIT 189. The total number of orbitals in a shell with principal quantum number n is (c) n2 (b What Is The Total Number Of Orbitals Associated With N=3 The third electron shell has 3 subshells, which are 3s, 3p, and 3d. An s subshell only has one orbital. To calculate the maximum number of electrons in each energy. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. A p subshell has three. For n = 3 there are nine orbitals, for n = 4 there are. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
The total number of orbitals in an energy level designated by principal quantum number n is equal to What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The third electron shell has 3 subshells, which are 3s, 3p, and 3d. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
Total number of orbitals associated with principal quantum number n = 3 is 6 What Is The Total Number Of Orbitals Associated With N=3 A p subshell has three. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is. What Is The Total Number Of Orbitals Associated With N=3.
From www.toppr.com
The total number of orbitals in a shell with the principal quantum number n is What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. An. What Is The Total Number Of Orbitals Associated With N=3.
From lucillemeowdurham.blogspot.com
The Size of an Atomic Orbital Is Associated With What Is The Total Number Of Orbitals Associated With N=3 For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. The total number of orbitals possible for a given principal quantum number n can be calculated using. What Is The Total Number Of Orbitals Associated With N=3.
From drivenheisenberg.blogspot.com
Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The 3rd Electron Of The N Atom What Is The Total Number Of Orbitals Associated With N=3 Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: To calculate the maximum number of electrons in each energy. The. What Is The Total Number Of Orbitals Associated With N=3.
From quantumechanicscourse.weebly.com
Chemistry Orbitals What Is The Total Number Of Orbitals Associated With N=3 \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. To calculate the maximum number of electrons in each energy. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. The total number of orbitals in the n =. What Is The Total Number Of Orbitals Associated With N=3.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is The Total Number Of Orbitals Associated With N=3 The third electron shell has 3 subshells, which are 3s, 3p, and 3d. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. An s subshell only has one. What Is The Total Number Of Orbitals Associated With N=3.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Practice Problems YouTube What Is The Total Number Of Orbitals Associated With N=3 For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. An s subshell only has one orbital. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. The total number of orbitals in the n = 4 principal shell is the sum of the. What Is The Total Number Of Orbitals Associated With N=3.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica What Is The Total Number Of Orbitals Associated With N=3 Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. To calculate the maximum number of electrons in each energy. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on.. What Is The Total Number Of Orbitals Associated With N=3.
From www.youtube.com
What is the maximum number of orbitals that can be identified with the following quantum numbers What Is The Total Number Of Orbitals Associated With N=3 For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. A p subshell has three. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is. What Is The Total Number Of Orbitals Associated With N=3.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is The Total Number Of Orbitals Associated With N=3 To calculate the maximum number of electrons in each energy. A p subshell has three. The total number of orbitals possible for a given principal quantum number n can be calculated using the formula. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal. What Is The Total Number Of Orbitals Associated With N=3.
From askfilo.com
The number of orbitals associated with quantum numbers n=5,ms =+21 Quest.. What Is The Total Number Of Orbitals Associated With N=3 A p subshell has three. The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. An s subshell only has one orbital. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and. What Is The Total Number Of Orbitals Associated With N=3.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a unique set of quantum What Is The Total Number Of Orbitals Associated With N=3 The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. A p subshell has three. To calculate the maximum number of electrons in each energy. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. An s. What Is The Total Number Of Orbitals Associated With N=3.
From in.pinterest.com
Azimuthal quantum number. Teaching chemistry, Science facts, Quantum What Is The Total Number Of Orbitals Associated With N=3 For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are 0 to n − 1 and. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and. What Is The Total Number Of Orbitals Associated With N=3.
From www.youtube.com
TOP NEET JEE CET NCERT Question Total number orbitals associated with Principal quantum no n= 3 What Is The Total Number Of Orbitals Associated With N=3 To calculate the maximum number of electrons in each energy. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. Each wavefunction with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. For any value of principal quantum number (n), the possible values of azimuthal quantum number (l) are. What Is The Total Number Of Orbitals Associated With N=3.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts What Is The Total Number Of Orbitals Associated With N=3 Thus, we find a total of 16 orbitals in the n = 4 shell of an atom. The third electron shell has 3 subshells, which are 3s, 3p, and 3d. \( \mathop 1\limits_{(l = 0)} + \mathop 3\limits_{(l = 1)} +. For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n =. What Is The Total Number Of Orbitals Associated With N=3.
From www.youtube.com
What is the total number of orbitals associated with the principal quantum number n = 3? YouTube What Is The Total Number Of Orbitals Associated With N=3 The total number of orbitals in the n = 4 principal shell is the sum of the number of orbitals in each subshell and is equal to n 2: The number of orbitals for a given principal quantum number n can be calculated using the formula @$\begin {align*}n^2\end {align*}@$. A p subshell has three. Each wavefunction with an allowed combination. What Is The Total Number Of Orbitals Associated With N=3.