Ideal Gas Isochoric Heating . Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric process, the volume of the gas remains constant. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. isochoric process in ideal gases. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. What is an ideal gas. For example, suppose you have an ideal gas in a closed rigid container;
from www.numerade.com
For example, suppose you have an ideal gas in a closed rigid container; calculate the specific heat of an ideal gas for either an isobaric or isochoric process. isochoric process in ideal gases. Explain the difference between the heat capacities of an. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). What is an ideal gas. in an isochoric process, the volume of the gas remains constant.
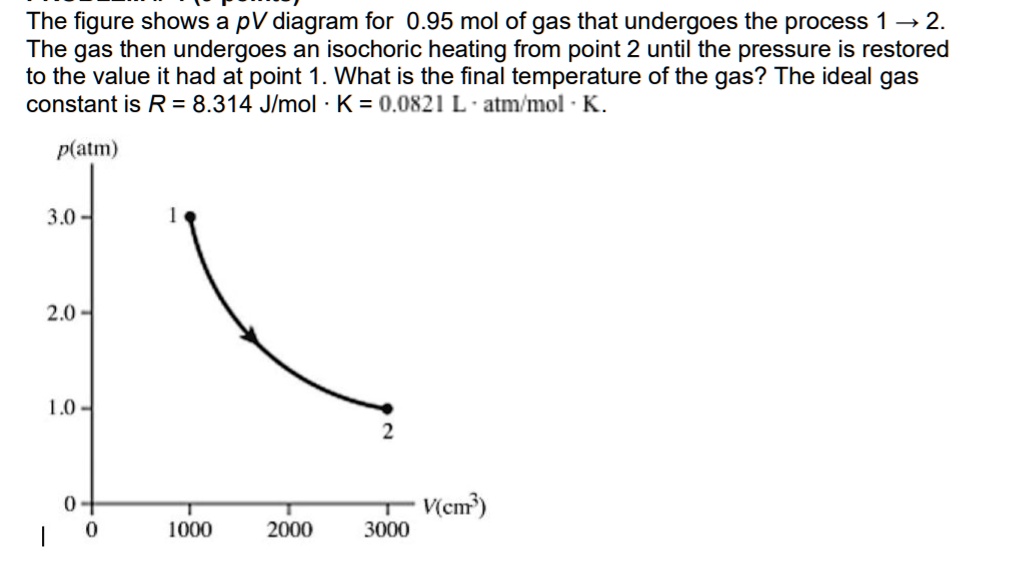
The figure shows a pV diagram for 0.95 mol of gas that undergoes the
Ideal Gas Isochoric Heating the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. Explain the difference between the heat capacities of an. For example, suppose you have an ideal gas in a closed rigid container; in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. isochoric process in ideal gases. What is an ideal gas. in an isochoric process, the volume of the gas remains constant.
From www.numerade.com
The figure shows a pV diagram for 0.95 mol of gas that undergoes the Ideal Gas Isochoric Heating calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). isochoric process in ideal gases. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v). Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED A heat engine follows a square cycle, as shown, with an Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. calculate the specific heat of an. Ideal Gas Isochoric Heating.
From www.doubtnut.com
One mole of ideal gas undergoes following cyclic process (i) Isochoric Ideal Gas Isochoric Heating isochoric process in ideal gases. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. For example, suppose you have an ideal gas in a closed rigid container; Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its. Ideal Gas Isochoric Heating.
From www.studocu.com
Processes of Ideal Gas Processes of Ideal Gas Isometric or Isochoric Ideal Gas Isochoric Heating the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). For example, suppose you have an ideal gas in a closed rigid container; isochoric process in ideal gases. Explain the difference between the heat capacities of an. in an isochoric system, three moles of. Ideal Gas Isochoric Heating.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas Isochoric Heating For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. For example, suppose you have an ideal gas in a closed rigid container; calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Explain the difference between the heat capacities of an. in an. Ideal Gas Isochoric Heating.
From commons.wikimedia.org
FileIsobaric, isochoric and isothermal process in ideal gas.png Ideal Gas Isochoric Heating in an isochoric process, the volume of the gas remains constant. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). What is an ideal gas. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston. Ideal Gas Isochoric Heating.
From www.toppr.com
One mole of an ideal gas undergoes the following cyclic process(i Ideal Gas Isochoric Heating For example, suppose you have an ideal gas in a closed rigid container; in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. in an isochoric process, the volume of the gas remains constant. What is an ideal gas. Explain the difference between the heat capacities of an. . Ideal Gas Isochoric Heating.
From holooly.com
A heat engine using a monatomic ideal gas goes through the following Ideal Gas Isochoric Heating isochoric process in ideal gases. Explain the difference between the heat capacities of an. in an isochoric process, the volume of the gas remains constant. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For example, suppose you have an ideal gas in a closed rigid container; For an ideal gas,. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED A monatomic ideal gas undergoes a cyclical process as shown in Ideal Gas Isochoric Heating isochoric process in ideal gases. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. What is an ideal gas. in an isochoric process, the volume of the gas remains constant. For example, suppose you have an ideal gas in a closed rigid container; in an isochoric system, three moles of. Ideal Gas Isochoric Heating.
From www.slideshare.net
Revision on thermodynamics Ideal Gas Isochoric Heating What is an ideal gas. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. For example, suppose you have an ideal gas in a closed. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED A diatomic ideal gas acts as a heat engine following a process Ideal Gas Isochoric Heating For example, suppose you have an ideal gas in a closed rigid container; calculate the specific heat of an ideal gas for either an isobaric or isochoric process. in an isochoric process, the volume of the gas remains constant. What is an ideal gas. the work done by an ideal gas is defined as its pressure times. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVEDOne mole of an ideal gas undergoes the following cyclic process Ideal Gas Isochoric Heating What is an ideal gas. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). isochoric process in ideal gases.. Ideal Gas Isochoric Heating.
From www.numerade.com
The figure shows a pV diagram for 0.95 mol of gas that undergoes the Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. What is an ideal gas. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For example, suppose you have an ideal gas in a closed. Ideal Gas Isochoric Heating.
From www.doubtnut.com
One mole of ideal monatomic gas was taken through isochoric heating fr Ideal Gas Isochoric Heating in an isochoric process, the volume of the gas remains constant. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. isochoric process in ideal gases. Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its pressure times its change in. Ideal Gas Isochoric Heating.
From www.chegg.com
Solved Consider a heat engine cycle consisting of isochoric Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. in an isochoric process, the volume of the gas remains constant. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. For example, suppose you have an ideal gas in a closed rigid container; calculate the specific heat of an. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED 1 gmol of an ideal gas forms the following cycle in a closed Ideal Gas Isochoric Heating in an isochoric process, the volume of the gas remains constant. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. in an isochoric system, three moles of hydrogen gas is trapped inside. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED 3 Entropy of Isochoric Process monoatomic ideal gas undergoes Ideal Gas Isochoric Heating For example, suppose you have an ideal gas in a closed rigid container; in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. in an isochoric process, the volume of the gas remains constant. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is. Ideal Gas Isochoric Heating.
From joe-mccullough.com
Heat and Thermodynamics Ideal Gas Isochoric Heating in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. For example, suppose you have an ideal gas in a closed rigid container; in an isochoric process, the volume of the gas remains constant. What is an ideal gas. the work done by an ideal gas is defined. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVEDA diatomic ideal gas acts as heat engine following process Ideal Gas Isochoric Heating isochoric process in ideal gases. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. What is an ideal gas. in an isochoric process, the volume of the gas remains constant. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. . Ideal Gas Isochoric Heating.
From www.chegg.com
Solved An ideal gas heat engine has two isochoric and two Ideal Gas Isochoric Heating What is an ideal gas. in an isochoric process, the volume of the gas remains constant. For example, suppose you have an ideal gas in a closed rigid container; calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the work done by an ideal gas is defined as its pressure times. Ideal Gas Isochoric Heating.
From www.toppr.com
An ideal gas is taken through a complete cycle in three steps Ideal Gas Isochoric Heating in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. Explain the difference between the heat capacities of an. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. For example, suppose you have an ideal gas in a closed rigid. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED The figure shows a pV diagram for 0.98 mol of an ideal gas that Ideal Gas Isochoric Heating in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv).. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED 3 PV diagram cycle An ideal gas undergoes the following four Ideal Gas Isochoric Heating For example, suppose you have an ideal gas in a closed rigid container; the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. in an. Ideal Gas Isochoric Heating.
From fr.slideserve.com
PPT Thermodynamics Temperature, Heat Transfer, and First Law of Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For example, suppose you have an ideal gas in a closed rigid container; the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v. Ideal Gas Isochoric Heating.
From www.doubtnut.com
An ideal gas undergoes isothermal expansion followed by heat removel a Ideal Gas Isochoric Heating the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). Explain the difference between the heat capacities of an. in an isochoric process, the volume of the gas remains constant. isochoric process in ideal gases. in an isochoric system, three moles of hydrogen. Ideal Gas Isochoric Heating.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Ideal Gas Isochoric Heating What is an ideal gas. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. isochoric process in ideal gases. Explain the difference between the heat capacities of an. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. the work done. Ideal Gas Isochoric Heating.
From www.slideserve.com
PPT Work and Heat Readings Chapter 17 PowerPoint Presentation, free Ideal Gas Isochoric Heating What is an ideal gas. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. in an isochoric process, the volume of the gas remains constant. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. For an ideal gas, the relationship between. Ideal Gas Isochoric Heating.
From www.youtube.com
Isochoric Process Thermodynamics Work, Heat & Internal Energy, PV Ideal Gas Isochoric Heating isochoric process in ideal gases. What is an ideal gas. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the work done by an ideal gas is defined as its pressure. Ideal Gas Isochoric Heating.
From slideplayer.com
Heat, work, isothermal and ppt download Ideal Gas Isochoric Heating calculate the specific heat of an ideal gas for either an isobaric or isochoric process. Explain the difference between the heat capacities of an. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric system, three moles of hydrogen gas is. Ideal Gas Isochoric Heating.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint Ideal Gas Isochoric Heating in an isochoric process, the volume of the gas remains constant. For example, suppose you have an ideal gas in a closed rigid container; What is an ideal gas. isochoric process in ideal gases. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. calculate the specific. Ideal Gas Isochoric Heating.
From questions.kunduz.com
For an ideal gas four fundamental process... Physical Chemistry Ideal Gas Isochoric Heating What is an ideal gas. For example, suppose you have an ideal gas in a closed rigid container; For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. Explain the difference. Ideal Gas Isochoric Heating.
From www.youtube.com
Ideal Isometric (Isochoric) Process On Piston (Find Change In Internal Ideal Gas Isochoric Heating For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. calculate the specific heat of an ideal gas for either an isobaric or isochoric process. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). What. Ideal Gas Isochoric Heating.
From slideplayer.com
FLUID MECHANICS LECTURE ppt download Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. isochoric process in ideal gases. the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). in an isochoric process, the volume of the gas remains constant. What is an ideal gas. in an isochoric. Ideal Gas Isochoric Heating.
From www.slideserve.com
PPT Chapter 9 Energy, Enthalpy and Thermochemistry PowerPoint Ideal Gas Isochoric Heating Explain the difference between the heat capacities of an. What is an ideal gas. For example, suppose you have an ideal gas in a closed rigid container; in an isochoric system, three moles of hydrogen gas is trapped inside an enclosed container with a piston on. For an ideal gas, the relationship between pressure (p), temperature (t), and volume. Ideal Gas Isochoric Heating.
From www.numerade.com
SOLVED The isobaricisochoric cycle of an ideal diatomic gas is Ideal Gas Isochoric Heating the work done by an ideal gas is defined as its pressure times its change in volume, or p δ v (or pdv). What is an ideal gas. For an ideal gas, the relationship between pressure (p), temperature (t), and volume (v) is given by the ideal. in an isochoric process, the volume of the gas remains constant.. Ideal Gas Isochoric Heating.