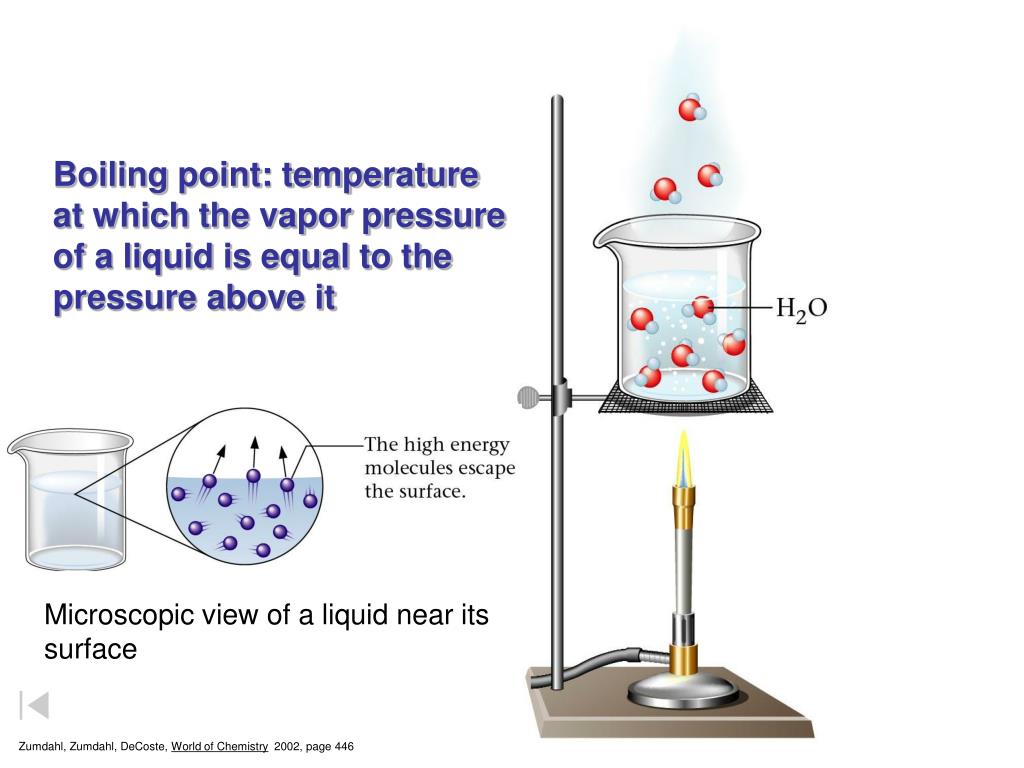
What Affects Vapor Pressure For Liquids . As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. Vapor pressure is defined as the partial pressure of a substance in the. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Will the vapor pressure of the liquid increase or decrease? At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure.
from www.slideserve.com
Vapor pressure is defined as the partial pressure of a substance in the. Will the vapor pressure of the liquid increase or decrease? Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapor pressure is defined as the partial pressure of a substance in the. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. Will the vapor pressure of the liquid increase or decrease? The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Affects Vapor Pressure For Liquids At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Will the vapor pressure of the liquid increase or decrease?. What Affects Vapor Pressure For Liquids.
From webmis.highland.cc.il.us
Vapor Pressure What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. The. What Affects Vapor Pressure For Liquids.
From www.numerade.com
SOLVED For liquids, which of the following factors affect vapor What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5347387 What Affects Vapor Pressure For Liquids As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. The vapor pressure of a liquid is the equilibrium pressure of. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Vapor Pressure of Solutions PowerPoint Presentation, free What Affects Vapor Pressure For Liquids At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is defined as the partial pressure of a substance in the. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Vapor pressure is. What Affects Vapor Pressure For Liquids.
From worksheets.ekocraft-appleleaf.com
Liquid Pressure Worksheet Worksheets For Kindergarten What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. What Affects Vapor Pressure For Liquids.
From chemistryskills.com
Define and explain vapour pressure Chemistry Skills What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Another important property of liquids (and solids) that is governed. What Affects Vapor Pressure For Liquids.
From www.youtube.com
factors affecting vapour pressure of a liquid solution chemistry What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the. What Affects Vapor Pressure For Liquids.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. It turns out that having a nonvolatile solute in the. What Affects Vapor Pressure For Liquids.
From www.shutterstock.com
8,060 Vapor pressure Images, Stock Photos & Vectors Shutterstock What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Vapor pressure is defined as the partial pressure of a substance in the. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The vapor. What Affects Vapor Pressure For Liquids.
From www.youtube.com
Vapor pressure, factors affecting and dynamic equilibrium Fsc 4th What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Affects Vapor Pressure For Liquids At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is defined as the partial pressure of a substance in the. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the. What Affects Vapor Pressure For Liquids.
From classnotes.org.in
Vapour Pressure Chemistry, Class 11, States of Matter What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they. What Affects Vapor Pressure For Liquids.
From socratic.org
Explain how each of the following affects the vapour pressure of a What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Vapor pressure. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,.. What Affects Vapor Pressure For Liquids.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Will the vapor pressure of the liquid increase or decrease? Another. What Affects Vapor Pressure For Liquids.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Affects Vapor Pressure For Liquids Vapor pressure is defined as the partial pressure of a substance in the. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. Will the vapor pressure of the liquid increase or decrease? As the temperature of a liquid increases, the vapor pressure of. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID3042045 What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Will the vapor pressure of the liquid increase or decrease? Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. At the normal boiling point of a liquid, the vapor pressure is equal to the. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Unit 3 Phase Changes & Energy PowerPoint Presentation ID3721071 What Affects Vapor Pressure For Liquids As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a. What Affects Vapor Pressure For Liquids.
From www.youtube.com
vapour pressure of liquids solution solutions class 12 chemistry What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Will the vapor pressure of the liquid increase or decrease? Vapor pressure is defined as the partial. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation What Affects Vapor Pressure For Liquids At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of. What Affects Vapor Pressure For Liquids.
From study.com
Understanding How Intermolecular Forces Affect Vapor Pressure What Affects Vapor Pressure For Liquids At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is defined as the partial pressure of a substance in the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Another important property of. What Affects Vapor Pressure For Liquids.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Affects Vapor Pressure For Liquids As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. The pressure exerted by the gas in equilibrium with a solid or liquid in. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5347387 What Affects Vapor Pressure For Liquids Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Affects Vapor Pressure For Liquids Will the vapor pressure of the liquid increase or decrease? The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. Vapor pressure is defined as the partial pressure of a substance in the. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Affects Vapor Pressure For Liquids Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. Will the vapor pressure of the liquid increase or decrease? Vapor pressure is defined as the partial pressure of a substance in the. That is, the pressure of the vapor resulting from evaporation of. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint What Affects Vapor Pressure For Liquids The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. It turns out that having. What Affects Vapor Pressure For Liquids.
From www.chegg.com
Solved For liquids, which of the factors affect vapor What Affects Vapor Pressure For Liquids Vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed system at. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the. That is, the pressure of the vapor resulting from evaporation of a liquid. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5347387 What Affects Vapor Pressure For Liquids That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure. What Affects Vapor Pressure For Liquids.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Affects Vapor Pressure For Liquids The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Will the vapor pressure of the liquid increase or decrease? That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At the normal boiling point of a liquid, the vapor pressure is equal to. What Affects Vapor Pressure For Liquids.
From byjus.com
What is the effect on vapour pressure of solution when external What Affects Vapor Pressure For Liquids The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. Vapor pressure is defined as the partial pressure of a substance in the. Another important property. What Affects Vapor Pressure For Liquids.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Affects Vapor Pressure For Liquids Will the vapor pressure of the liquid increase or decrease? That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It turns out that having a nonvolatile solute in the liquid will decrease the. What Affects Vapor Pressure For Liquids.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Affects Vapor Pressure For Liquids The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [1] 760 torr,. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container. What Affects Vapor Pressure For Liquids.