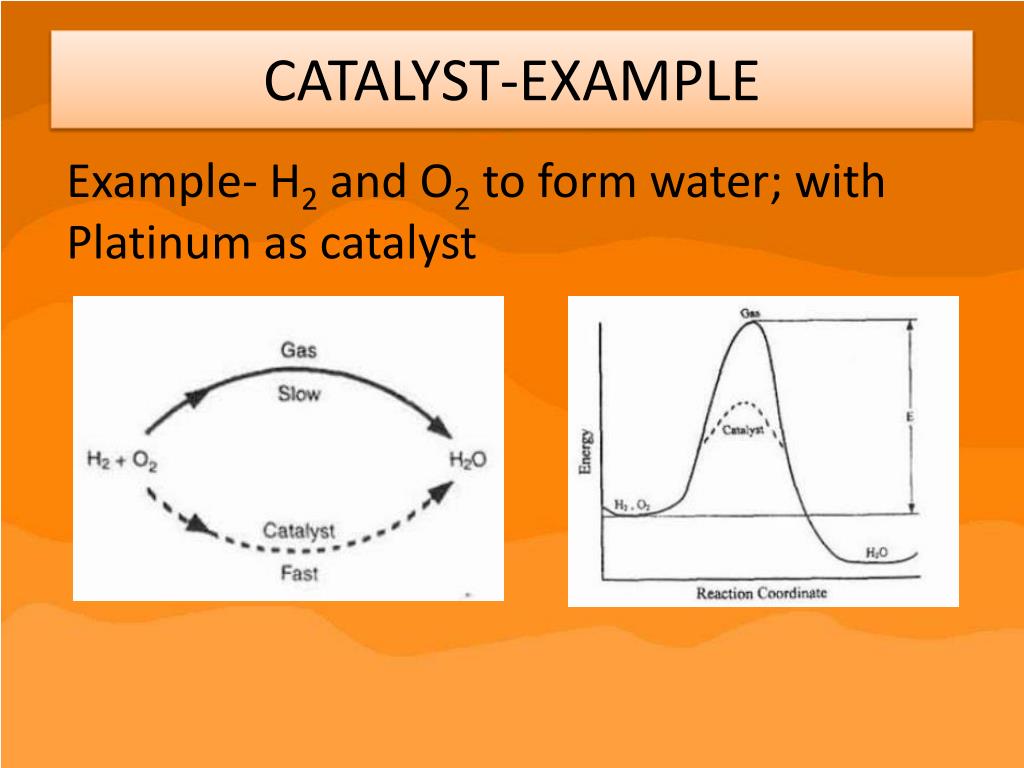
Catalyst Technology Meaning . Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. The substance that plays the key role. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. Enzymes are naturally occurring catalysts responsible for many. A tool for optimising processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions.
from www.slideserve.com
The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Enzymes are naturally occurring catalysts responsible for many. A tool for optimising processes. The substance that plays the key role. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalyst Technology Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. The substance that plays the key role. A tool for optimising processes. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Technology Meaning The substance that plays the key role. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Enzymes are naturally occurring catalysts responsible for many. A tool for optimising processes.. Catalyst Technology Meaning.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction. Catalyst Technology Meaning.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Technology Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant. Catalyst Technology Meaning.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Technology Meaning A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Enzymes are naturally occurring catalysts responsible for many. Simply. Catalyst Technology Meaning.
From www.researchgate.net
Classification of catalyst. Download Scientific Diagram Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. A tool. Catalyst Technology Meaning.
From www.researchgate.net
Scheme illustration of the proposed catalyst design strategy for Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. A tool for optimising processes. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. The meaning of catalyst is a. Catalyst Technology Meaning.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID676158 Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. Enzymes are naturally occurring catalysts responsible for many. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A tool for optimising. Catalyst Technology Meaning.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst Technology Meaning A tool for optimising processes. The substance that plays the key role. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the. Catalyst Technology Meaning.
From www.slideshare.net
Catalyst Catalyst Technology Meaning A tool for optimising processes. The substance that plays the key role. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Catalysis is a process that provides a new way to a chemical. Catalyst Technology Meaning.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 Catalyst Technology Meaning The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. Catalyst Technology Meaning.
From omaryouthbradley.blogspot.com
What is a Catalyst Catalyst Technology Meaning A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. The substance that plays the key role. Catalysis. Catalyst Technology Meaning.
From www.powerblanket.com
What is Selective Catalytic Reduction? Powerblanket Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to. Catalyst Technology Meaning.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Technology Meaning A tool for optimising processes. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. The substance that. Catalyst Technology Meaning.
From www.catalyticcombustion.com
Introduction To Catalyst Technology inar Catalytic Combustion Catalyst Technology Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The substance that plays the key role. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that speeds up a chemical reaction, or lowers the. Catalyst Technology Meaning.
From www.slideshare.net
Catalytic Reforming Catalyst, Process Technology and Operations Ove… Catalyst Technology Meaning The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. The substance that plays the key role. A tool for optimising processes. Catalyst, in. Catalyst Technology Meaning.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Technology Meaning A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. Simply put, a. Catalyst Technology Meaning.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Technology Meaning A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Enzymes are naturally occurring catalysts responsible for many. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalyst,. Catalyst Technology Meaning.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID1563031 Catalyst Technology Meaning Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A. Catalyst Technology Meaning.
From www.tersusdef.com
Selective Catalytic Reduction How it works Tersus Diesel Exhaust Catalyst Technology Meaning Enzymes are naturally occurring catalysts responsible for many. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.. Catalyst Technology Meaning.
From mavink.com
Catalytic Cracking Diagram Catalyst Technology Meaning A tool for optimising processes. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Enzymes are naturally occurring catalysts responsible for many. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically.. Catalyst Technology Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Technology Meaning A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate. Catalyst Technology Meaning.
From www.lummustechnology.com
Catalysts Lummus Technology Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The substance that plays the key role. A tool for optimising processes.. Catalyst Technology Meaning.
From www.slideshare.net
The importance of catalyst Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst binds to a reactant and it increases the number. Catalyst Technology Meaning.
From www.aecc.eu
AECC Catalysts From oxidation catalysts to threeway catalysts Catalyst Technology Meaning The substance that plays the key role. A tool for optimising processes. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A. Catalyst Technology Meaning.
From www.mdpi.com
Catalysts Free FullText Industrial Applications of Enzymes Recent Catalyst Technology Meaning A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to. Catalyst Technology Meaning.
From www.slideshare.net
Catalytic Reforming Catalyst, Process Technology and Operations Ove… Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or. Catalyst Technology Meaning.
From www.lct.ugent.be
Catalyst design Laboratory for Chemical Technology Catalyst Technology Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant. Catalyst Technology Meaning.
From www.researchgate.net
(PDF) SINGLESITE CATALYST TECHNOLOGY & POLYOLEFIN MARKETS IN TRANSITION Catalyst Technology Meaning A tool for optimising processes. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. Enzymes are naturally. Catalyst Technology Meaning.
From www.thoughtco.com
Catalysts and How They Work Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A tool for optimising processes. Simply put, a catalyst is a substance. Catalyst Technology Meaning.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Technology Meaning A tool for optimising processes. Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the. Catalyst Technology Meaning.
From www.researchgate.net
The catalyst technology pipeline. Download Scientific Diagram Catalyst Technology Meaning Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that speeds up a chemical reaction, or lowers. Catalyst Technology Meaning.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Technology Meaning A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst is a substance that speeds up a chemical reaction, or lowers the. Catalyst Technology Meaning.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. A tool for optimising processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under. Catalyst Technology Meaning.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Technology Meaning Catalysis is a process that provides a new way to a chemical reaction with the lowest activation energy. The substance that plays the key role. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A tool for optimising processes. Simply put, a catalyst is a substance that increases the rate of a reaction. Catalyst Technology Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Technology Meaning A tool for optimising processes. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Simply put, a catalyst is a substance that increases the rate of a reaction without altering the products of the reaction and is unchanged. The substance that plays the key role. Catalyst,. Catalyst Technology Meaning.