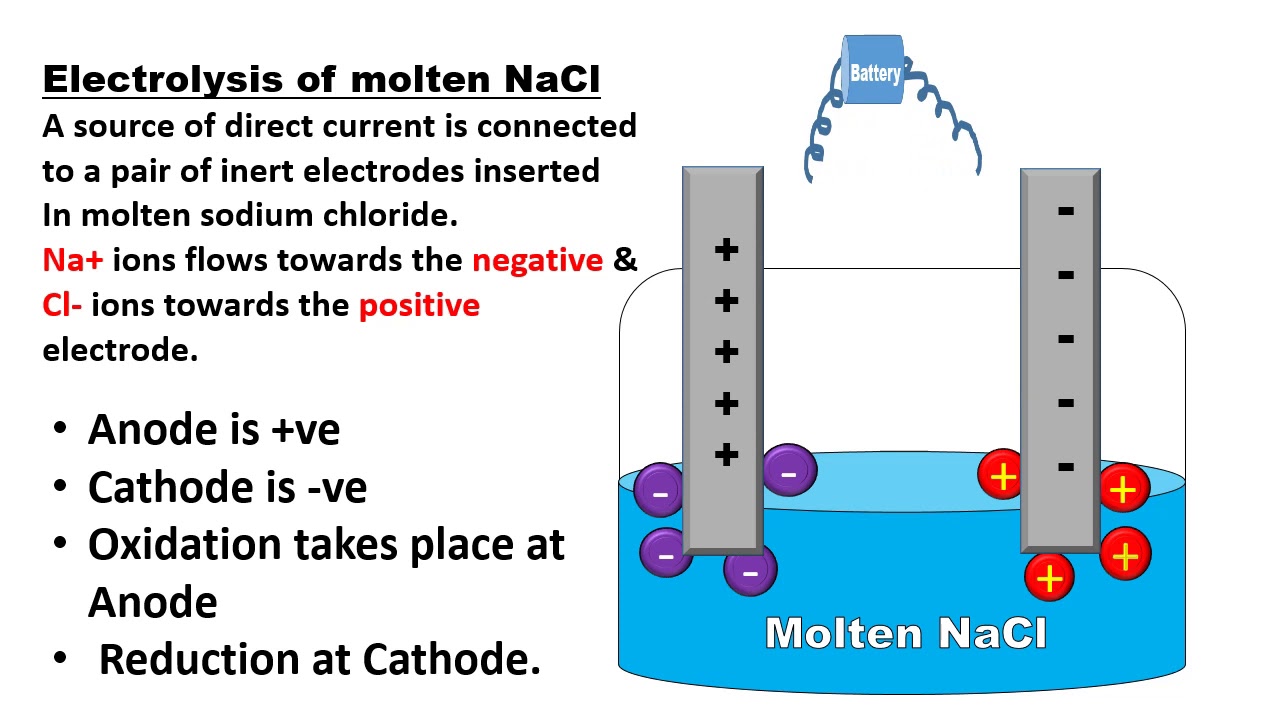
How Do Electrolytes Work Chemistry . Electrolyte balance is crucial to many body functions. In order to conduct a current, a. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. They also contribute to energy. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. A substance that, when added to water, renders it conductive, is known as an electrolyte. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). These are nutritionally called macrominerals. A common example of an. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract.
from madisonmeowmercado.blogspot.com
Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. These are nutritionally called macrominerals. A substance that, when added to water, renders it conductive, is known as an electrolyte. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). Electrolyte balance is crucial to many body functions. A common example of an. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. In order to conduct a current, a. They also contribute to energy.
Anode and Cathode in Electrolysis
How Do Electrolytes Work Chemistry Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). These are nutritionally called macrominerals. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A common example of an. In order to conduct a current, a. Electrolyte balance is crucial to many body functions. A substance that, when added to water, renders it conductive, is known as an electrolyte. They also contribute to energy. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses.
From etn.news
Electrolyte Enabling ions transport within a Liion battery How Do Electrolytes Work Chemistry Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. These are nutritionally called macrominerals. In order to conduct a current, a. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. A common example of an. Electrolyte balance is crucial to many body functions. They also. How Do Electrolytes Work Chemistry.
From healthinpics.blogspot.com
The Roles of Electrolytes How Do Electrolytes Work Chemistry Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A substance that, when added to water, renders it conductive, is known as an electrolyte. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. These are nutritionally called macrominerals. An electrolyte is a compound that conducts an electric current when it. How Do Electrolytes Work Chemistry.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki How Do Electrolytes Work Chemistry Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. A substance that, when added to water, renders it conductive, is known as an electrolyte.. How Do Electrolytes Work Chemistry.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID6585303 How Do Electrolytes Work Chemistry They also contribute to energy. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolyte balance is crucial to many body functions. These are nutritionally called macrominerals. In order to conduct a current, a. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Your cells. How Do Electrolytes Work Chemistry.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors How Do Electrolytes Work Chemistry Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. A substance that, when added to water, renders it conductive, is known as an electrolyte. In order to conduct a current, a. A common example of an. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and. How Do Electrolytes Work Chemistry.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes How Do Electrolytes Work Chemistry Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. In order to conduct a current, a. Electrolyte balance is crucial to many body functions. These are nutritionally called macrominerals. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. A substance that, when added to water, renders it conductive, is known. How Do Electrolytes Work Chemistry.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes How Do Electrolytes Work Chemistry In order to conduct a current, a. A common example of an. They also contribute to energy. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte, in chemistry and physics, substance that conducts electric current. How Do Electrolytes Work Chemistry.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts How Do Electrolytes Work Chemistry These are nutritionally called macrominerals. Electrolyte balance is crucial to many body functions. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. In order to conduct a current, a. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate. How Do Electrolytes Work Chemistry.
From www.youtube.com
Strong Electrolytes YouTube How Do Electrolytes Work Chemistry Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. Electrolyte balance is crucial to many body functions. A common example of an. An electrolyte. How Do Electrolytes Work Chemistry.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo How Do Electrolytes Work Chemistry Electrolyte balance is crucial to many body functions. In order to conduct a current, a. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). A common example of an. Electrolyte,. How Do Electrolytes Work Chemistry.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation How Do Electrolytes Work Chemistry Electrolyte balance is crucial to many body functions. A common example of an. A substance that, when added to water, renders it conductive, is known as an electrolyte. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively. How Do Electrolytes Work Chemistry.
From pandai.me
Electrolytic Cell How Do Electrolytes Work Chemistry In order to conduct a current, a. A substance that, when added to water, renders it conductive, is known as an electrolyte. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of. How Do Electrolytes Work Chemistry.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free How Do Electrolytes Work Chemistry The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and. How Do Electrolytes Work Chemistry.
From www.worksheetsplanet.com
What is an Electrolyte Definition of Electrolyte How Do Electrolytes Work Chemistry They also contribute to energy. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. The primary electrolytes required in the body fluid are cations. How Do Electrolytes Work Chemistry.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis How Do Electrolytes Work Chemistry These are nutritionally called macrominerals. Electrolyte balance is crucial to many body functions. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). In order to conduct a current, a. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte,. How Do Electrolytes Work Chemistry.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes How Do Electrolytes Work Chemistry An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. They also contribute to energy. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates,. How Do Electrolytes Work Chemistry.
From www.osmosis.org
Overview of Electrolyte Balance Osmosis Video Library How Do Electrolytes Work Chemistry The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. These are nutritionally called macrominerals. A substance that, when added to water, renders it conductive, is. How Do Electrolytes Work Chemistry.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download How Do Electrolytes Work Chemistry A common example of an. A substance that, when added to water, renders it conductive, is known as an electrolyte. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. These are nutritionally called macrominerals. An electrolyte is a compound that conducts. How Do Electrolytes Work Chemistry.
From energyeducation.ca
Electrolyte Energy Education How Do Electrolytes Work Chemistry A common example of an. Electrolyte balance is crucial to many body functions. A substance that, when added to water, renders it conductive, is known as an electrolyte. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at. How Do Electrolytes Work Chemistry.
From www.slideserve.com
PPT Ionic Conductivity and Solid Electrolytes I The Basics How Do Electrolytes Work Chemistry In order to conduct a current, a. A substance that, when added to water, renders it conductive, is known as an electrolyte. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolyte balance is crucial to. How Do Electrolytes Work Chemistry.
From www.mysportscience.com
Electrolytes under investigation How Do Electrolytes Work Chemistry These are nutritionally called macrominerals. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). They also contribute to energy. In order to conduct a current, a. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. An electrolyte is a. How Do Electrolytes Work Chemistry.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps How Do Electrolytes Work Chemistry Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte balance is crucial to many body functions. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A substance that, when added to water, renders it conductive, is known as an electrolyte. The primary electrolytes required in the body fluid are. How Do Electrolytes Work Chemistry.
From www.freepik.com
Electrolyte Images Free Vectors, Stock Photos & PSD How Do Electrolytes Work Chemistry An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of. How Do Electrolytes Work Chemistry.
From www.istockphoto.com
Electrolyte Illustrations, RoyaltyFree Vector Graphics & Clip Art iStock How Do Electrolytes Work Chemistry An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. A substance that, when added to water, renders it conductive, is known as an electrolyte. They also contribute to energy. These are nutritionally called macrominerals. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte balance. How Do Electrolytes Work Chemistry.
From www.slideshare.net
CLINICAL CHEMISTRY ELECTROLYTES How Do Electrolytes Work Chemistry In order to conduct a current, a. These are nutritionally called macrominerals. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative. How Do Electrolytes Work Chemistry.
From www.pinterest.com
What is Electrolysis and Electrolyte with examples. Teaching How Do Electrolytes Work Chemistry They also contribute to energy. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. These are nutritionally called macrominerals. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive. How Do Electrolytes Work Chemistry.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation ID5686615 How Do Electrolytes Work Chemistry The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). Electrolyte balance is crucial to many body functions. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward. How Do Electrolytes Work Chemistry.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Do Electrolytes Work Chemistry The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A common example of an. An electrolyte is a compound that conducts an electric current when it is in an aqueous. How Do Electrolytes Work Chemistry.
From www.youtube.com
Electrolytes Definition, Examples, & Practice YouTube How Do Electrolytes Work Chemistry These are nutritionally called macrominerals. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). A substance that, when added to water, renders it conductive, is known as an electrolyte. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. Electrolyte. How Do Electrolytes Work Chemistry.
From www.electricity-magnetism.org
How do electrolytes conduct electricity? How Do Electrolytes Work Chemistry A common example of an. An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. In order to conduct a current, a. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A. How Do Electrolytes Work Chemistry.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary How Do Electrolytes Work Chemistry Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate. How Do Electrolytes Work Chemistry.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts How Do Electrolytes Work Chemistry A substance that, when added to water, renders it conductive, is known as an electrolyte. In order to conduct a current, a. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. Electrolyte balance is crucial to many body functions. Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte, in. How Do Electrolytes Work Chemistry.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace How Do Electrolytes Work Chemistry Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. A. How Do Electrolytes Work Chemistry.
From mammothmemory.net
Electrolyte particles in the body transmitting electricity How Do Electrolytes Work Chemistry A substance that, when added to water, renders it conductive, is known as an electrolyte. Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. The primary electrolytes required in the body fluid are cations (of calcium, potassium, sodium, and magnesium) and anions (of chloride, carbonates, aminoacetates, phosphates, and iodide). Electrolyte balance is crucial to many. How Do Electrolytes Work Chemistry.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Do Electrolytes Work Chemistry Electrolytes help to regulate water distribution, govern acid base balance and transmit nerve impulses. Electrolyte, in chemistry and physics, substance that conducts electric current as a result of a dissociation into positively and negatively charged particles called ions, which migrate toward and ordinarily are discharged at the negative and positive terminals (cathode and anode) of an electric circuit, respectively. The. How Do Electrolytes Work Chemistry.