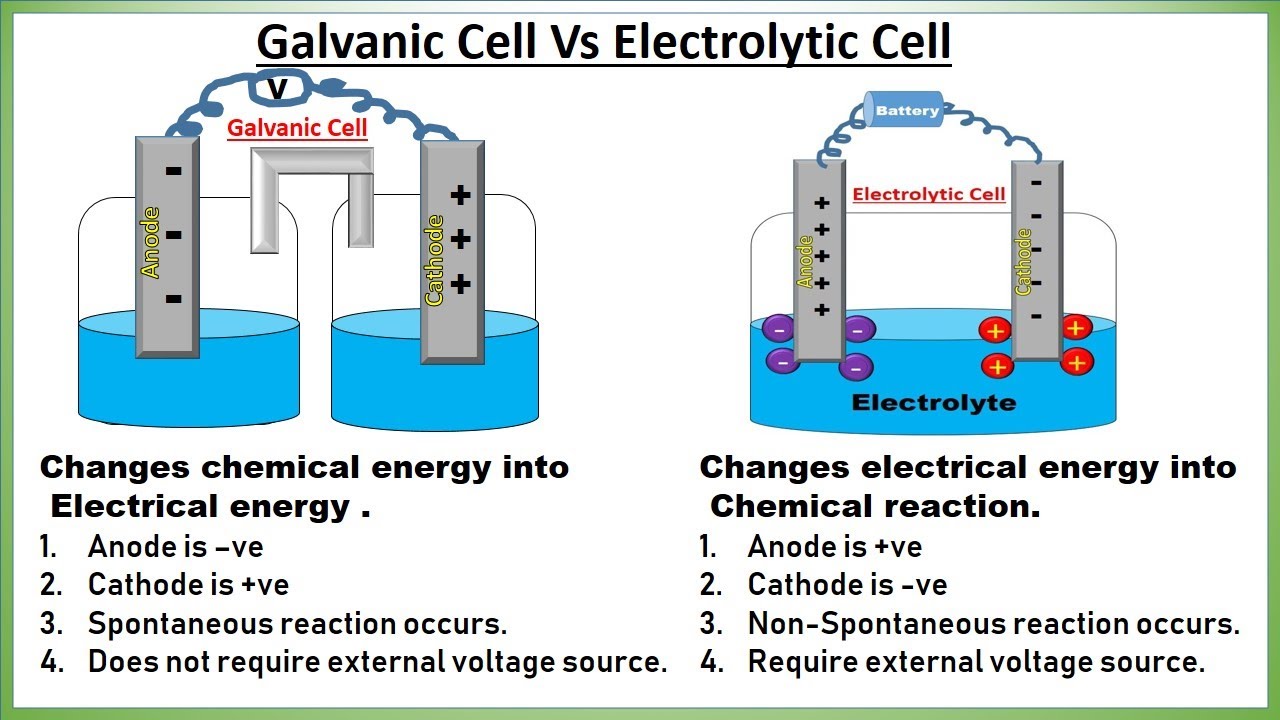
Electrochemical Vs Galvanic Cell . devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. galvanic cells are electrochemical cells that can be used to do work. Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from.
from www.youtube.com
a galvanic cell is an electrochemical cell in which spontaneous. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. galvanic cells are electrochemical cells that can be used to do work. Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur.
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells
Electrochemical Vs Galvanic Cell galvanic cells are electrochemical cells that can be used to do work. galvanic cells are electrochemical cells that can be used to do work. Figure 19.3.3 shows a typical galvanic cell that uses the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell is an electrochemical cell in which spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. galvanic cells are electrochemical cells that can be used to do work. a galvanic cell is an electrochemical cell in which spontaneous. Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic (voltaic). Electrochemical Vs Galvanic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Electrochemical Vs Galvanic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort. Electrochemical Vs Galvanic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Figure 19.3.3 shows a typical galvanic. Electrochemical Vs Galvanic Cell.
From www.vecteezy.com
Electrochemical cell or Galvanic cell with Voltmeter and the function Electrochemical Vs Galvanic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. galvanic cells are electrochemical. Electrochemical Vs Galvanic Cell.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. galvanic cells are electrochemical cells that can be used to do work. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to. Electrochemical Vs Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. galvanic cells are electrochemical cells that can be used to do work. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. devices. Electrochemical Vs Galvanic Cell.
From sites.google.com
week 914 chemistry Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. galvanic cells are electrochemical. Electrochemical Vs Galvanic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. devices of this sort. Electrochemical Vs Galvanic Cell.
From www.studocu.com
Galvanic VS Electrolytic CELL SUMMARY CHAPTER 3 ELECTROCHEMISTRY Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. Electrochemical Vs Galvanic Cell.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses Electrochemical Vs Galvanic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. galvanic cells are electrochemical cells that can be used to do work. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an. Electrochemical Vs Galvanic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. galvanic cells are electrochemical cells that can be used to do work. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts. Electrochemical Vs Galvanic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. galvanic cells are electrochemical cells that can be used to do work. devices. Electrochemical Vs Galvanic Cell.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemical Vs Galvanic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic cell is an electrochemical. Electrochemical Vs Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the. Electrochemical Vs Galvanic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Electrochemical Vs Galvanic Cell galvanic cells are electrochemical cells that can be used to do work. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate. Electrochemical Vs Galvanic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell is an electrochemical cell in which spontaneous. galvanic cells are electrochemical cells that can be used to do work. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction. Electrochemical Vs Galvanic Cell.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell. Electrochemical Vs Galvanic Cell.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Electrochemical Vs Galvanic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell is an electrochemical cell in which spontaneous. Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell, or. Electrochemical Vs Galvanic Cell.
From llivetolearn.blogspot.com
Live to Learn, Learn to Live Electrochemical cells, Difference between Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. galvanic cells are electrochemical cells that. Electrochemical Vs Galvanic Cell.
From www.pinterest.es
What are the differences between Galvanic cell and Electrolytic cell Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. the galvanic cell, or. Electrochemical Vs Galvanic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. galvanic cells are electrochemical cells that can be used to do work. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the. Electrochemical Vs Galvanic Cell.
From mygreexampreparation.com
Galvanic vs Electrolytic Cell MCAT (Electrochemistry Guide) Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate. Electrochemical Vs Galvanic Cell.
From www.pinterest.pt
Galvanic Cell and Electrolytic Cell Electrochemical Cells Teaching Electrochemical Vs Galvanic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses. Electrochemical Vs Galvanic Cell.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. devices of this sort are generally referred to as electrochemical cells, and those in which. Electrochemical Vs Galvanic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Vs Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the. Electrochemical Vs Galvanic Cell.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram Electrochemical Vs Galvanic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort. Electrochemical Vs Galvanic Cell.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Electrochemical Vs Galvanic Cell galvanic cells are electrochemical cells that can be used to do work. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell is an electrochemical cell in which spontaneous. Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell,. Electrochemical Vs Galvanic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Figure 19.3.3 shows a typical galvanic cell that uses the. devices of this sort. Electrochemical Vs Galvanic Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Electrochemical Vs Galvanic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. galvanic cells are electrochemical cells that can be used to do work. a galvanic cell. Electrochemical Vs Galvanic Cell.
From www.dreamstime.com
Electrochemical Cell or Galvanic Cell, the Daniell Cell Stock Vector Electrochemical Vs Galvanic Cell a galvanic cell is an electrochemical cell in which spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. devices of this sort. Electrochemical Vs Galvanic Cell.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic (voltaic) cell uses the. Electrochemical Vs Galvanic Cell.
From saylordotorg.github.io
Electrochemistry Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. a galvanic cell is an electrochemical cell in which spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an. Electrochemical Vs Galvanic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. a galvanic cell is an electrochemical cell in which spontaneous. galvanic cells are electrochemical cells that can be used to do work. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell,. Electrochemical Vs Galvanic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic. Electrochemical Vs Galvanic Cell.
From www.vrogue.co
Galvanic Cell Vs Electrolytic Cell Animation Electroc vrogue.co Electrochemical Vs Galvanic Cell Figure 19.3.3 shows a typical galvanic cell that uses the. galvanic cells are electrochemical cells that can be used to do work. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the. Electrochemical Vs Galvanic Cell.