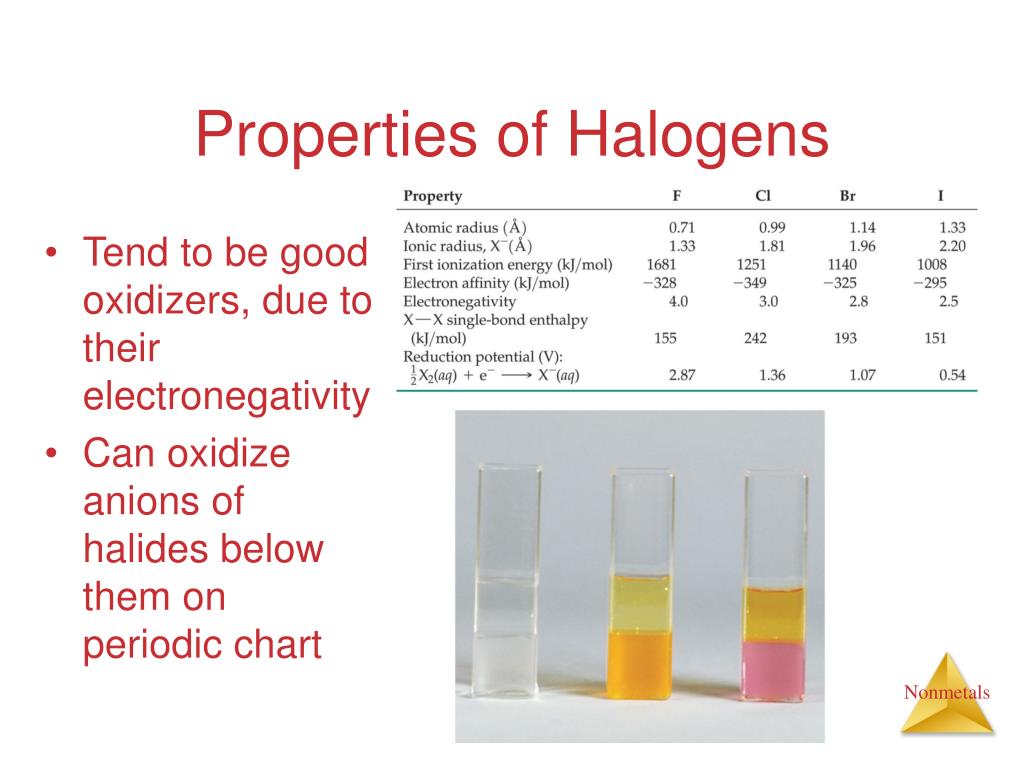
Properties Of Halogens Atomic Radius . sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. Discusses trends in atomic radius, electronegativity, electron affinity and. properties of the halogens. Group 17 can be found in the table’s 17th. physical properties of the halogens. Placed in a vertical column on. The halogens make up group 17 of the elements on the periodic table. (b) covalent radii of the elements are shown. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. atomic and physical properties. in this section, we will explore some core trends in the properties of the halogens. in (a) the atomic radius for the halogens increases down the group as n increases. We will cover electronegativity, boiling point, and solubility of.
from exoicnyjk.blob.core.windows.net
in (a) the atomic radius for the halogens increases down the group as n increases. We will cover electronegativity, boiling point, and solubility of. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. Group 17 can be found in the table’s 17th. in this section, we will explore some core trends in the properties of the halogens. atomic and physical properties. The halogens make up group 17 of the elements on the periodic table. physical properties of the halogens. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. (b) covalent radii of the elements are shown.
Properties Of Halogens Gas at Lora Maynes blog
Properties Of Halogens Atomic Radius physical properties of the halogens. We will cover electronegativity, boiling point, and solubility of. (b) covalent radii of the elements are shown. atomic and physical properties. Placed in a vertical column on. in (a) the atomic radius for the halogens increases down the group as n increases. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. The halogens make up group 17 of the elements on the periodic table. physical properties of the halogens. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. in this section, we will explore some core trends in the properties of the halogens. properties of the halogens. Group 17 can be found in the table’s 17th. Discusses trends in atomic radius, electronegativity, electron affinity and.
From pediabay.com
Atomic Radius of Elements (With Periodic table Chart) Pediabay Properties Of Halogens Atomic Radius atomic and physical properties. in (a) the atomic radius for the halogens increases down the group as n increases. properties of the halogens. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. physical properties of the halogens. Placed in a vertical column on. in this section,. Properties Of Halogens Atomic Radius.
From periodictableguide.com
Where are the Halogens located on the Periodic table? Properties Of Halogens Atomic Radius atomic and physical properties. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. The halogens make up group 17 of the elements on the periodic table. Placed in a vertical column on. Group 17 can be found in the table’s 17th. physical properties of the halogens. properties of. Properties Of Halogens Atomic Radius.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Properties Of Halogens Atomic Radius Discusses trends in atomic radius, electronegativity, electron affinity and. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. We will cover electronegativity, boiling point, and solubility of. The halogens make up group 17 of the elements on the periodic table. properties. Properties Of Halogens Atomic Radius.
From www.vedantu.com
Halogens Learn Definition, Properties, Facts & Examples Properties Of Halogens Atomic Radius Group 17 can be found in the table’s 17th. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. Placed in a vertical column on. in this section, we will explore some core trends in the properties of the halogens. The halogens make up group 17 of the elements on the. Properties Of Halogens Atomic Radius.
From chemistrypage.in
Halogen Elements Why Halogen are So Reactive Elemental Nature Properties Of Halogens Atomic Radius The halogens make up group 17 of the elements on the periodic table. Group 17 can be found in the table’s 17th. We will cover electronegativity, boiling point, and solubility of. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. properties. Properties Of Halogens Atomic Radius.
From maddisonbarrett.z21.web.core.windows.net
Chart Of Atomic Radius Properties Of Halogens Atomic Radius The halogens make up group 17 of the elements on the periodic table. in (a) the atomic radius for the halogens increases down the group as n increases. Placed in a vertical column on. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at). Properties Of Halogens Atomic Radius.
From slidesplayer.com
Chapter 16 The pblock elements (Ⅲ) ppt download Properties Of Halogens Atomic Radius Placed in a vertical column on. atomic and physical properties. We will cover electronegativity, boiling point, and solubility of. in (a) the atomic radius for the halogens increases down the group as n increases. Discusses trends in atomic radius, electronegativity, electron affinity and. atomic radius (increases down the group) the size of the nucleus increases down a. Properties Of Halogens Atomic Radius.
From collegedunia.com
Halogens Properties, Electronic Configuration & Characteristics Properties Of Halogens Atomic Radius sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. atomic and physical properties. in (a) the atomic radius for the halogens increases down the group as n increases. (b) covalent radii of the elements are shown. Group 17 can be found in the table’s 17th. in this section,. Properties Of Halogens Atomic Radius.
From www.chegg.com
Solved Properties of the Alkall Metals& Halogens Atomic Properties Of Halogens Atomic Radius Placed in a vertical column on. Group 17 can be found in the table’s 17th. (b) covalent radii of the elements are shown. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. in this section, we will explore some core trends in the properties of the halogens. atomic radius. Properties Of Halogens Atomic Radius.
From slideplayer.com
Electronic Structure of Atoms ppt download Properties Of Halogens Atomic Radius We will cover electronegativity, boiling point, and solubility of. (b) covalent radii of the elements are shown. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. atomic and physical properties. Group 17 can be found in the table’s 17th. in. Properties Of Halogens Atomic Radius.
From www.youtube.com
The properties and reactions of the halogens YouTube Properties Of Halogens Atomic Radius atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. properties of the halogens. The halogens make up group 17 of the elements on the periodic table. atomic and physical properties. (b) covalent radii of the elements are shown. in. Properties Of Halogens Atomic Radius.
From www.vedantu.com
Halogens Learn Definition, Properties, Facts & Examples Properties Of Halogens Atomic Radius sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. physical properties of the halogens. Placed in a vertical column on. in this section, we will explore some core trends in the properties of the halogens. properties of the halogens. atomic radius (increases down the group) the size. Properties Of Halogens Atomic Radius.
From askfilo.com
Fig. 3.4 (b) Variation of atomic radius with atomic number for alkali met.. Properties Of Halogens Atomic Radius sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. Group 17 can be found in the table’s 17th. The halogens make up group 17 of the elements on the periodic table. in this section, we will explore some core trends in the properties of the halogens. We will cover electronegativity,. Properties Of Halogens Atomic Radius.
From exotrdzdb.blob.core.windows.net
One Chemical Property Of Halogens at Edward Kroll blog Properties Of Halogens Atomic Radius We will cover electronegativity, boiling point, and solubility of. Group 17 can be found in the table’s 17th. Placed in a vertical column on. Discusses trends in atomic radius, electronegativity, electron affinity and. physical properties of the halogens. atomic and physical properties. in this section, we will explore some core trends in the properties of the halogens.. Properties Of Halogens Atomic Radius.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation ID1275374 Properties Of Halogens Atomic Radius The halogens make up group 17 of the elements on the periodic table. in this section, we will explore some core trends in the properties of the halogens. We will cover electronegativity, boiling point, and solubility of. atomic and physical properties. properties of the halogens. Discusses trends in atomic radius, electronegativity, electron affinity and. physical properties. Properties Of Halogens Atomic Radius.
From chemistnotes.com
Halogens General Characteristics & Physical properties Chemistry Notes Properties Of Halogens Atomic Radius (b) covalent radii of the elements are shown. atomic and physical properties. in (a) the atomic radius for the halogens increases down the group as n increases. Group 17 can be found in the table’s 17th. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. The halogens make up. Properties Of Halogens Atomic Radius.
From ecampusontario.pressbooks.pub
8.7 Periodic Trends and Variation of Properties General Chemistry Properties Of Halogens Atomic Radius atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. properties of the halogens. in this section, we will explore some core trends. Properties Of Halogens Atomic Radius.
From exotrdzdb.blob.core.windows.net
One Chemical Property Of Halogens at Edward Kroll blog Properties Of Halogens Atomic Radius in this section, we will explore some core trends in the properties of the halogens. Group 17 can be found in the table’s 17th. (b) covalent radii of the elements are shown. physical properties of the halogens. The halogens make up group 17 of the elements on the periodic table. sections below cover the trends in atomic. Properties Of Halogens Atomic Radius.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2411422 Properties Of Halogens Atomic Radius atomic and physical properties. in this section, we will explore some core trends in the properties of the halogens. Placed in a vertical column on. (b) covalent radii of the elements are shown. properties of the halogens. Group 17 can be found in the table’s 17th. sections below cover the trends in atomic radius, electronegativity, electron. Properties Of Halogens Atomic Radius.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Properties Of Halogens Atomic Radius atomic and physical properties. The halogens make up group 17 of the elements on the periodic table. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling. Properties Of Halogens Atomic Radius.
From exotrdzdb.blob.core.windows.net
One Chemical Property Of Halogens at Edward Kroll blog Properties Of Halogens Atomic Radius Discusses trends in atomic radius, electronegativity, electron affinity and. properties of the halogens. physical properties of the halogens. We will cover electronegativity, boiling point, and solubility of. The halogens make up group 17 of the elements on the periodic table. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,.. Properties Of Halogens Atomic Radius.
From exotfbtmr.blob.core.windows.net
Chemical Properties Of Halogen Group at Edward Cunningham blog Properties Of Halogens Atomic Radius physical properties of the halogens. The halogens make up group 17 of the elements on the periodic table. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. in (a) the atomic radius for the halogens increases down the group as n increases. properties of the halogens. in. Properties Of Halogens Atomic Radius.
From www.youtube.com
Physical properties of halogens YouTube Properties Of Halogens Atomic Radius Placed in a vertical column on. We will cover electronegativity, boiling point, and solubility of. The halogens make up group 17 of the elements on the periodic table. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. atomic radius (increases down the group) the size of the nucleus increases down. Properties Of Halogens Atomic Radius.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6702617 Properties Of Halogens Atomic Radius in (a) the atomic radius for the halogens increases down the group as n increases. physical properties of the halogens. in this section, we will explore some core trends in the properties of the halogens. Placed in a vertical column on. (b) covalent radii of the elements are shown. properties of the halogens. atomic and. Properties Of Halogens Atomic Radius.
From scienceinfo.com
Chemical Properties of Halogen Elements and Hydrogen Halides Properties Of Halogens Atomic Radius The halogens make up group 17 of the elements on the periodic table. (b) covalent radii of the elements are shown. in (a) the atomic radius for the halogens increases down the group as n increases. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. We will cover electronegativity, boiling. Properties Of Halogens Atomic Radius.
From www.jove.com
Periodic Properties of Elements Halogens JoVE Book Properties Of Halogens Atomic Radius atomic and physical properties. (b) covalent radii of the elements are shown. in (a) the atomic radius for the halogens increases down the group as n increases. physical properties of the halogens. properties of the halogens. We will cover electronegativity, boiling point, and solubility of. in this section, we will explore some core trends in. Properties Of Halogens Atomic Radius.
From exoicnyjk.blob.core.windows.net
Properties Of Halogens Gas at Lora Maynes blog Properties Of Halogens Atomic Radius sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. in this section, we will explore some core trends in the properties of the halogens. atomic and physical properties. Discusses trends in atomic radius, electronegativity, electron affinity and. atomic radius (increases down the group) the size of the nucleus. Properties Of Halogens Atomic Radius.
From newtondesk.com
Halogens (Periodic Table) Properties, Uses, & Facts Properties Of Halogens Atomic Radius The halogens make up group 17 of the elements on the periodic table. atomic and physical properties. in this section, we will explore some core trends in the properties of the halogens. Placed in a vertical column on. We will cover electronegativity, boiling point, and solubility of. Group 17 can be found in the table’s 17th. atomic. Properties Of Halogens Atomic Radius.
From www.nagwa.com
Question Video Determining How the Atomic Radius and Reactivity of the Properties Of Halogens Atomic Radius sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl < br < i < at) because the. Discusses trends in atomic radius, electronegativity, electron affinity and. The halogens make up group 17. Properties Of Halogens Atomic Radius.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.3.2 Chemical Properties Halogens & Hydrogen Properties Of Halogens Atomic Radius We will cover electronegativity, boiling point, and solubility of. The halogens make up group 17 of the elements on the periodic table. in (a) the atomic radius for the halogens increases down the group as n increases. (b) covalent radii of the elements are shown. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and. Properties Of Halogens Atomic Radius.
From bradleyrahman.z13.web.core.windows.net
Chart Of Atomic Radius Of Elements Properties Of Halogens Atomic Radius physical properties of the halogens. Group 17 can be found in the table’s 17th. in this section, we will explore some core trends in the properties of the halogens. atomic and physical properties. Placed in a vertical column on. in (a) the atomic radius for the halogens increases down the group as n increases. Discusses trends. Properties Of Halogens Atomic Radius.
From www.breakingatom.com
Group 17 The Halogens Properties Of Halogens Atomic Radius We will cover electronegativity, boiling point, and solubility of. atomic and physical properties. in (a) the atomic radius for the halogens increases down the group as n increases. Placed in a vertical column on. physical properties of the halogens. atomic radius (increases down the group) the size of the nucleus increases down a group (f <. Properties Of Halogens Atomic Radius.
From periodicitychem.weebly.com
Atomic Radius Periodicity Project Properties Of Halogens Atomic Radius in this section, we will explore some core trends in the properties of the halogens. Group 17 can be found in the table’s 17th. in (a) the atomic radius for the halogens increases down the group as n increases. atomic radius (increases down the group) the size of the nucleus increases down a group (f < cl. Properties Of Halogens Atomic Radius.
From www.tes.com
AS Chemistry The Halogens (Trends in Physical & Chemical Properties Properties Of Halogens Atomic Radius We will cover electronegativity, boiling point, and solubility of. in this section, we will explore some core trends in the properties of the halogens. (b) covalent radii of the elements are shown. Placed in a vertical column on. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. physical properties. Properties Of Halogens Atomic Radius.
From chem.libretexts.org
Group 17 Physical Properties of the Halogens Chemistry LibreTexts Properties Of Halogens Atomic Radius physical properties of the halogens. sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility,. Discusses trends in atomic radius, electronegativity, electron affinity and. (b) covalent radii of the elements are shown. in this section, we will explore some core trends in the properties of the halogens. Placed in a. Properties Of Halogens Atomic Radius.