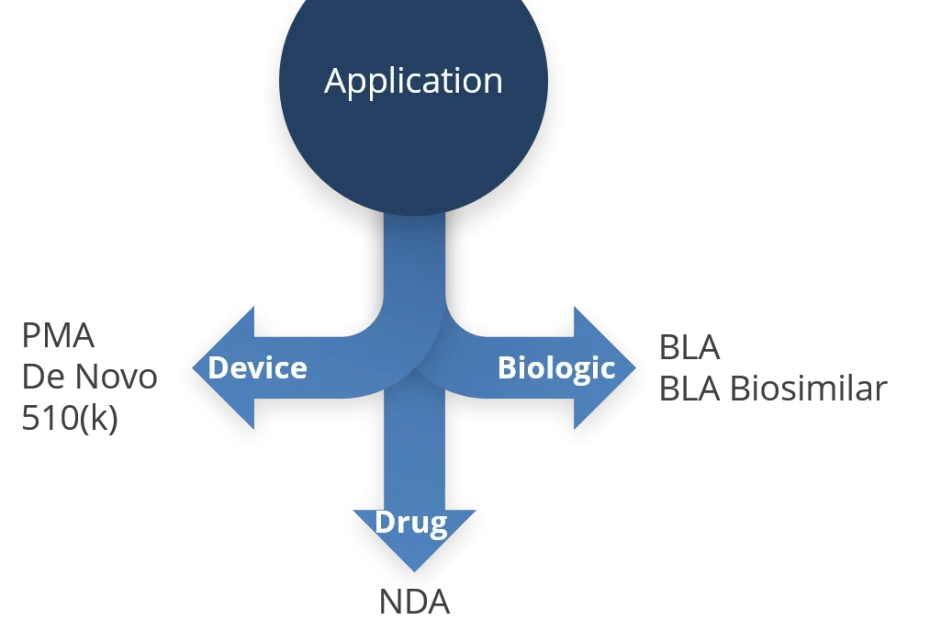
Combination Product Definition Fda . What is a combination product? Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Constituent part is a drug, device, or biological product that is part of a. Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. Full overview of the origin, definition and designation of combination products. The fda goes in to more detail: Combination product has the meaning set forth in § 3.2 (e) of this chapter. A combination product is a product composed of any combination of a drug and a device;
from www.avanti-europe.ch
Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and designation of combination products. Constituent part is a drug, device, or biological product that is part of a. What is a combination product? Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail: A combination product is a product composed of any combination of a drug and a device;
FDA submission routes for combination products easily explained
Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Regulatory review and evaluation of the safety. The fda goes in to more detail: Constituent part is a drug, device, or biological product that is part of a. A combination product is a product composed of any combination of a drug and a device; Full overview of the origin, definition and designation of combination products. What is a combination product? Combination product has the meaning set forth in § 3.2 (e) of this chapter. (1) a product comprised of two or more regulated.
From www.fda.gov
Combination Products FDA Combination Product Definition Fda Full overview of the origin, definition and designation of combination products. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Regulatory review and evaluation of the safety. What is a combination product? Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail:. Combination Product Definition Fda.
From medtechintelligence.com
Optimize Combination Products Select a Drug Delivery Device that Meets Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. Full overview of the origin, definition and designation of combination products. What is a combination product? Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. The fda goes in to more detail: Constituent part is a drug, device, or. Combination Product Definition Fda.
From www.kymanox.com
Combination Products Kymanox Combination Product Definition Fda Full overview of the origin, definition and designation of combination products. (1) a product comprised of two or more regulated. The fda goes in to more detail: Regulatory review and evaluation of the safety. What is a combination product? Combination product has the meaning set forth in § 3.2 (e) of this chapter. Food and drug administration (fda) defines combination. Combination Product Definition Fda.
From www.youtube.com
FDA cGMP Final Guidance for Combination Products l 21 CFR Part 4 Combination Product Definition Fda Full overview of the origin, definition and designation of combination products. Regulatory review and evaluation of the safety. What is a combination product? Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail: (1) a product comprised of two or more regulated. A combination product is a product composed. Combination Product Definition Fda.
From slidetodoc.com
Introduction to US FDA Regulatory Framework NSF SBIR Combination Product Definition Fda Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. What is a combination product? A combination product is a product composed of any combination of a drug and a device; Full overview of the origin, definition and designation of combination products. Constituent part is a drug, device, or biological product that is part. Combination Product Definition Fda.
From qbdworks.com
How To Apply QbD to Drug Device Combination Products Quality by Combination Product Definition Fda The fda goes in to more detail: Combination product has the meaning set forth in § 3.2 (e) of this chapter. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and designation of combination products. What is a combination product? A combination product is a product composed. Combination Product Definition Fda.
From www.slideshare.net
Combination Products Combination Product Definition Fda Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part of a. A combination product is a product composed of any combination of a drug and a device; What is a combination product? (1) a product comprised of two or more regulated. Food and drug administration (fda) defines combination products as. Combination Product Definition Fda.
From www.slideserve.com
PPT Cross Labeling Combination Products and User Fees PowerPoint Combination Product Definition Fda Full overview of the origin, definition and designation of combination products. A combination product is a product composed of any combination of a drug and a device; Combination product has the meaning set forth in § 3.2 (e) of this chapter. Constituent part is a drug, device, or biological product that is part of a. What is a combination product?. Combination Product Definition Fda.
From www.slideserve.com
PPT Regulatory Pathway for Platform Technologies PowerPoint Combination Product Definition Fda A combination product is a product composed of any combination of a drug and a device; Combination product has the meaning set forth in § 3.2 (e) of this chapter. What is a combination product? Constituent part is a drug, device, or biological product that is part of a. Full overview of the origin, definition and designation of combination products.. Combination Product Definition Fda.
From www.presentationeze.com
FDA Regulation Combination Products PresentationEZE Combination Product Definition Fda A combination product is a product composed of any combination of a drug and a device; The fda goes in to more detail: Constituent part is a drug, device, or biological product that is part of a. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and. Combination Product Definition Fda.
From ncogmp.com
FDAのFINAL GUIDANCE CGMP for combination products(201701) 西山経営研究所 Combination Product Definition Fda What is a combination product? Constituent part is a drug, device, or biological product that is part of a. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. (1) a product comprised of two or more regulated. A combination product is a product composed of any combination of a drug and a device;. Combination Product Definition Fda.
From www.avanti-europe.ch
FDA submission routes for combination products easily explained Combination Product Definition Fda Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part of a. Combination product has the meaning set forth in § 3.2 (e) of this chapter. What is a combination product? A combination product is a product composed of any combination of a drug and a device; Food and drug administration. Combination Product Definition Fda.
From www.youtube.com
Drug Device Combination Products Episode 07 GMP Requirements for Combination Product Definition Fda Full overview of the origin, definition and designation of combination products. Regulatory review and evaluation of the safety. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. (1) a product comprised of two or more regulated. Constituent part is a drug, device, or biological product that is part of a. Combination product has. Combination Product Definition Fda.
From drug-dev.com
FIXEDDOSE COMBINATIONS FixedDose Combination Products A Review Combination Product Definition Fda The fda goes in to more detail: What is a combination product? Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and designation of combination products. Constituent part is a drug,. Combination Product Definition Fda.
From www.slideshare.net
COMBINATION PRODUCTS Perspectives on FDA Regulation Combination Product Definition Fda A combination product is a product composed of any combination of a drug and a device; Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and designation of combination products. Regulatory review and evaluation of the safety. What is a combination product? (1) a product comprised of. Combination Product Definition Fda.
From www.ey.com
How to navigate EU regulations for drugdevice combination products Combination Product Definition Fda Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. A combination product is a product composed of any combination of a drug and a device; (1) a product comprised of two or more regulated. Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part. Combination Product Definition Fda.
From www.powershow.com
PPT FDA Guidance Drug Device Combination Products PowerPoint Combination Product Definition Fda The fda goes in to more detail: A combination product is a product composed of any combination of a drug and a device; Combination product has the meaning set forth in § 3.2 (e) of this chapter. What is a combination product? Constituent part is a drug, device, or biological product that is part of a. (1) a product comprised. Combination Product Definition Fda.
From angelanjohnson.com
DrugDevice Combination Treatments and Diagnostics Roundtable Angela Combination Product Definition Fda Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part of a. Full overview of the origin, definition and designation of combination products. The fda goes in to more detail: Combination product has the meaning set forth in § 3.2 (e) of this chapter. A combination product is a product composed. Combination Product Definition Fda.
From www.presentationeze.com
FDA Regulation Combination Products PresentationEZE Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. Full overview of the origin, definition and designation of combination products. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. What is a. Combination Product Definition Fda.
From www.slideserve.com
PPT FDA Regulation of Product Claims PowerPoint Presentation ID248341 Combination Product Definition Fda Regulatory review and evaluation of the safety. A combination product is a product composed of any combination of a drug and a device; (1) a product comprised of two or more regulated. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Constituent part is a drug, device, or biological product that is part. Combination Product Definition Fda.
From leanraqa.com
FDA Submission for Combination Products Regulatory + Quality Combination Product Definition Fda A combination product is a product composed of any combination of a drug and a device; (1) a product comprised of two or more regulated. Combination product has the meaning set forth in § 3.2 (e) of this chapter. Full overview of the origin, definition and designation of combination products. Constituent part is a drug, device, or biological product that. Combination Product Definition Fda.
From www.slideserve.com
PPT FDA Regulation of Combination Products PowerPoint Presentation Combination Product Definition Fda Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Regulatory review and evaluation of the safety. The fda goes in to more detail: A combination product is a product composed of any combination of a drug and a device; Constituent part is a drug, device, or biological product that is part of a.. Combination Product Definition Fda.
From operonstrategist.com
Challenges and Solutions in DrugDevice Combination Products Combination Product Definition Fda Constituent part is a drug, device, or biological product that is part of a. Full overview of the origin, definition and designation of combination products. A combination product is a product composed of any combination of a drug and a device; Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. What is a. Combination Product Definition Fda.
From www.presentationeze.com
FDA Regulation Combination Products PresentationEZE Combination Product Definition Fda What is a combination product? (1) a product comprised of two or more regulated. Full overview of the origin, definition and designation of combination products. The fda goes in to more detail: Combination product has the meaning set forth in § 3.2 (e) of this chapter. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that. Combination Product Definition Fda.
From operonstrategist.com
What Is Copackaged Combination Product? (Briefly Explained) Operon Combination Product Definition Fda A combination product is a product composed of any combination of a drug and a device; Combination product has the meaning set forth in § 3.2 (e) of this chapter. Full overview of the origin, definition and designation of combination products. The fda goes in to more detail: What is a combination product? Food and drug administration (fda) defines combination. Combination Product Definition Fda.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Combination Product Definition Fda Constituent part is a drug, device, or biological product that is part of a. Combination product has the meaning set forth in § 3.2 (e) of this chapter. Regulatory review and evaluation of the safety. A combination product is a product composed of any combination of a drug and a device; The fda goes in to more detail: Food and. Combination Product Definition Fda.
From emmainternational.com
FDA's New Compliance Program Combination Product Inspections Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail: Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part of a. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview. Combination Product Definition Fda.
From www.slideserve.com
PPT Combination Products Cross Labeling and Single Entity Labeling Combination Product Definition Fda The fda goes in to more detail: Regulatory review and evaluation of the safety. Full overview of the origin, definition and designation of combination products. Combination product has the meaning set forth in § 3.2 (e) of this chapter. (1) a product comprised of two or more regulated. What is a combination product? Constituent part is a drug, device, or. Combination Product Definition Fda.
From learngxp.com
3 Types of Combination Products You Should Know [Video] LearnGxP Combination Product Definition Fda The fda goes in to more detail: Full overview of the origin, definition and designation of combination products. Constituent part is a drug, device, or biological product that is part of a. (1) a product comprised of two or more regulated. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. A combination product. Combination Product Definition Fda.
From resource.ddregpharma.com
US FDA Safety Reporting Requirements for Combination Products Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail: Regulatory review and evaluation of the safety. What is a combination product? Full overview of the origin, definition and designation of combination products. A combination product is a product composed of any combination of a drug and a device;. Combination Product Definition Fda.
From www.kymanox.com
Combination Products Kymanox Combination Product Definition Fda Combination product has the meaning set forth in § 3.2 (e) of this chapter. Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Full overview of the origin, definition and designation of combination products. What is a combination product? Regulatory review and evaluation of the safety. The fda goes in to more detail:. Combination Product Definition Fda.
From www.presentationeze.com
FDA Regulation Combination Products PresentationEZE Combination Product Definition Fda Food and drug administration (fda) defines combination products as “therapeutic and diagnostic products that combine drugs,. Combination product has the meaning set forth in § 3.2 (e) of this chapter. The fda goes in to more detail: Constituent part is a drug, device, or biological product that is part of a. Regulatory review and evaluation of the safety. (1) a. Combination Product Definition Fda.
From www.presentationeze.com
US FDA Regulation of Combination products PresentationEZE Combination Product Definition Fda Constituent part is a drug, device, or biological product that is part of a. Full overview of the origin, definition and designation of combination products. A combination product is a product composed of any combination of a drug and a device; (1) a product comprised of two or more regulated. Regulatory review and evaluation of the safety. Combination product has. Combination Product Definition Fda.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Combination Product Definition Fda The fda goes in to more detail: Regulatory review and evaluation of the safety. Constituent part is a drug, device, or biological product that is part of a. Combination product has the meaning set forth in § 3.2 (e) of this chapter. Full overview of the origin, definition and designation of combination products. What is a combination product? (1) a. Combination Product Definition Fda.
From www.learngxp.com
MLV035 How the FDA Defines a Combination Product LearnGxP Combination Product Definition Fda Regulatory review and evaluation of the safety. (1) a product comprised of two or more regulated. Full overview of the origin, definition and designation of combination products. A combination product is a product composed of any combination of a drug and a device; Combination product has the meaning set forth in § 3.2 (e) of this chapter. What is a. Combination Product Definition Fda.