Titration Measurements . Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a type of chemical analysis. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. We consider it to be analysis because we use it to make a measurement. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. For example, you can use it to find the concentration of a solution of an acid.
from www.youtube.com
A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. We consider it to be analysis because we use it to make a measurement. Titration is a type of chemical analysis. For example, you can use it to find the concentration of a solution of an acid. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion.
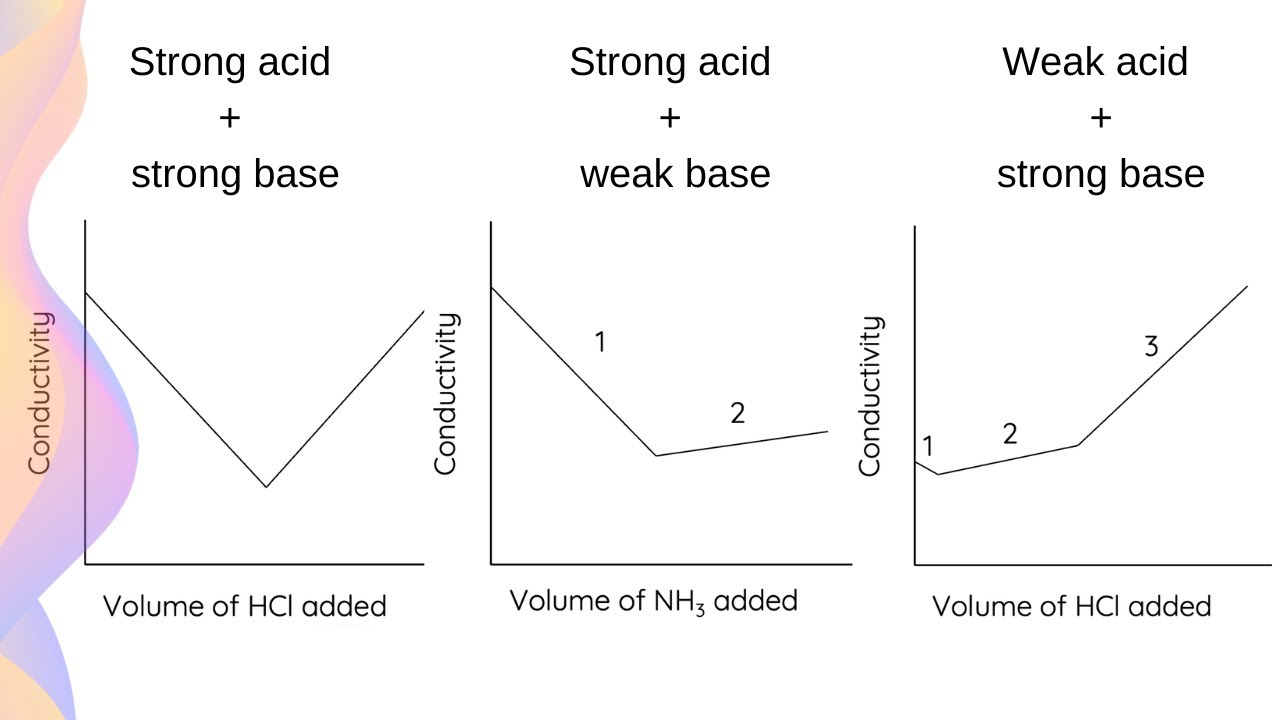
Conductometric Titration & Titration Curves // HSC Chemistry YouTube
Titration Measurements Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. We consider it to be analysis because we use it to make a measurement. Titration is a type of chemical analysis. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. For example, you can use it to find the concentration of a solution of an acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From www.researchgate.net
Charge titration measurements of HyA titrated with CTAB at different Titration Measurements Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion.. Titration Measurements.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Measurements We consider it to be analysis because we use it to make a measurement. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn about the titration. Titration Measurements.
From www.slideserve.com
PPT TITRATION CURVES PowerPoint Presentation ID1130069 Titration Measurements Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a. Titration Measurements.
From www.scribd.com
10 Precipitation Titration PDF Solubility Precipitation (Chemistry) Titration Measurements Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration. Titration Measurements.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Measurements Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a widely used technique to measure the amount of one substance present in another through. Titration Measurements.
From www.researchgate.net
Titration measurements to calculate the Kd values Analytical Titration Measurements Titration is a type of chemical analysis. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another. Titration Measurements.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Measurements It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. We consider. Titration Measurements.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Measurements Titration is a type of chemical analysis. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration, process of chemical analysis in which the quantity of some constituent. Titration Measurements.
From ck12.org
Titration CK12 Foundation Titration Measurements We consider it to be analysis because we use it to make a measurement. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. The basic process involves adding. Titration Measurements.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID6718194 Titration Measurements Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration, process of chemical analysis in which the quantity of some constituent of a sample. Titration Measurements.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Measurements Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Measurements.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Measurements It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. For example, you can use it to find the concentration of a solution of an acid. The basic process involves adding. Titration Measurements.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Titration Measurements Titration is a type of chemical analysis. We consider it to be analysis because we use it to make a measurement. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding. Titration Measurements.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset Titration Measurements We consider it to be analysis because we use it to make a measurement. Titration is a type of chemical analysis. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration Measurements.
From bramblechemistry.weebly.com
4C6 Titration Titration Measurements It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample. Titration Measurements.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Measurements Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration of a solution of an acid.. Titration Measurements.
From www.chemeurope.com
Titration Handbook Theory and Practice of Titration A guide to Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. For example, you can use it to find the concentration of a solution of an acid. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Learn about. Titration Measurements.
From www.researchgate.net
(A) ITC titration measurements of PVPBr (1.0 Â 10 À4 mol L À1 ) and Titration Measurements For example, you can use it to find the concentration of a solution of an acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn. Titration Measurements.
From gamma.app
Understanding Titration Titration Measurements Titration is a type of chemical analysis. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. We consider it to be analysis because we use it to make a measurement. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding. Titration Measurements.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Measurements Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a type of chemical analysis. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. Titration Measurements.
From www.science-revision.co.uk
Titrations Titration Measurements Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. We consider it to be analysis because we use it to make a measurement. Titration is a type of. Titration Measurements.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn about the titration technique through a practical experiment to determine the reacting. Titration Measurements.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. We consider it to be analysis because we use it to make a measurement. For example, you can use it to find the concentration of a solution of an acid. Titration is a type of chemical analysis.. Titration Measurements.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Titration Measurements.
From www.researchgate.net
(A) ITC titration measurements of PVPBr (1.0 × 10⁻⁴ mol L⁻¹) and ZnPc Titration Measurements For example, you can use it to find the concentration of a solution of an acid. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. We consider it to be analysis because we use it to make a measurement. Titration is a way of measuring the concentration of something,. Titration Measurements.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Measurements Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. We consider it to be analysis because we use it to make a measurement. Titration is a type of chemical analysis. The basic process involves adding a standard solution of one reagent to a known amount. Titration Measurements.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titration Measurements It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction.. Titration Measurements.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Measurements For example, you can use it to find the concentration of a solution of an acid. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction.. Titration Measurements.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Measurements Titration is a type of chemical analysis. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance. Titration Measurements.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a widely used technique to measure the amount of one substance present in another through a. Titration Measurements.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Titration Measurements Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to determine the concentration of a substance in a solution. For example, you can use it to find the concentration of a solution of an acid. Titration is a. Titration Measurements.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Measurements Titration is a type of chemical analysis. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration of a solution of an acid. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration Measurements.
From www.youtube.com
Titrations Percentage Uncertainty in Apparatus and Measurements YouTube Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in. Titration Measurements.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Titration Measurements Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration of a solution of an acid. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to. Titration Measurements.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Measurements The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration Measurements.