The Reaction Of Iron With Copper (Ii) Chloride Lab Answers . Also to determine the ratio. Take out the 100 ml beaker. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Add approximately 30 ml of the copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. To determine the number of moles of copper produced, the number of iron used up. How many moles of copper would be produced by reacting. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. This lab will be an attempt to get the highest possible percent yield in performing a single.
from www.youtube.com
Add approximately 30 ml of the copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Also to determine the ratio. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Take out the 100 ml beaker. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). This lab will be an attempt to get the highest possible percent yield in performing a single.
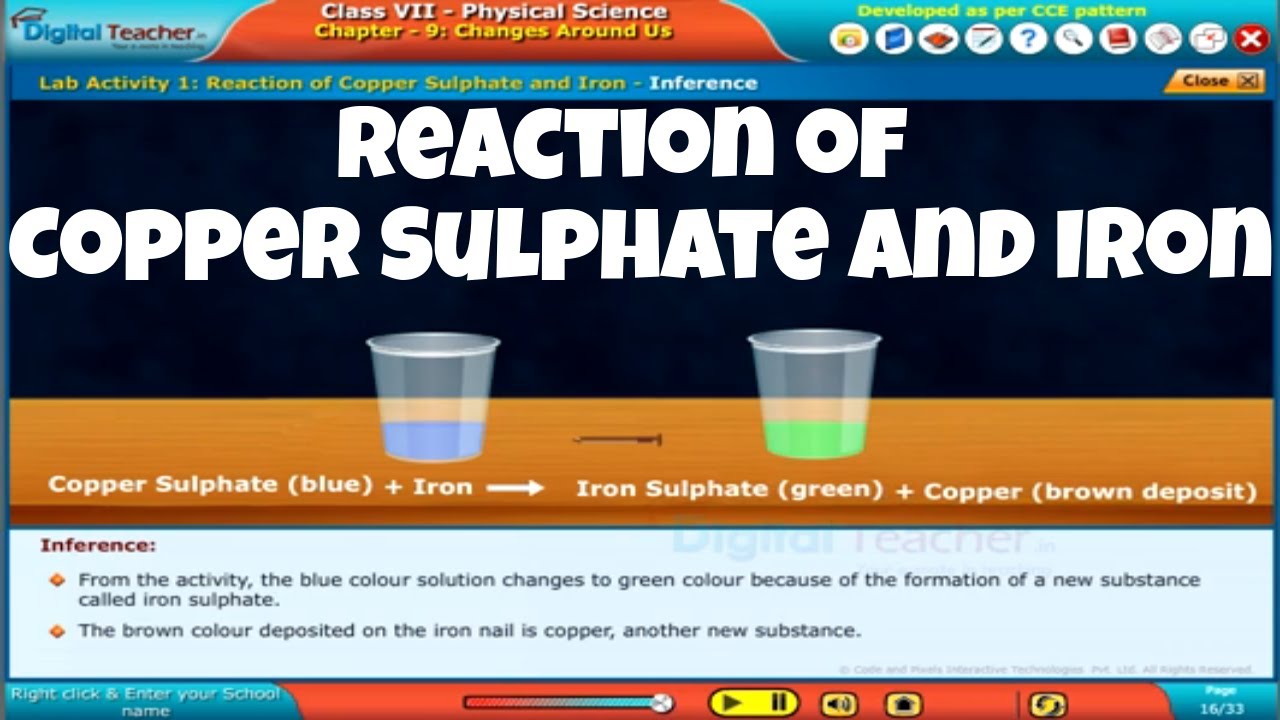
Lab Activity Reaction of Copper Sulphate and Iron, Class 7 Physics
The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. To determine the number of moles of copper produced, the number of iron used up. How many moles of copper would be produced by reacting. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Take out the 100 ml beaker. Also to determine the ratio. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. This lab will be an attempt to get the highest possible percent yield in performing a single. Add approximately 30 ml of the copper (ii) chloride.
From studylib.net
Aluminum Reaction with Copper (II) Chloride LAB The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. How many moles of copper would be produced by reacting. Write the complete balanced chemical equation for the reaction between copper (ii). The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.studocu.com
Lab 4 LAB 4 Stoichiometry of IronCopper (II) Sulfate Redox The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. How many moles of copper would be produced by reacting. Take out the 100 ml beaker. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Also to determine the ratio. In. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.numerade.com
SOLVED A solution is prepared by dissolving 63.8 grams of copper (II The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. To determine the number of moles of copper produced, the number of iron used up. How many moles of copper would be produced by reacting. This lab will be an attempt to get the highest possible. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.numerade.com
SOLVED If we have 0.572 grams of aluminum and 3.029 grams of copper(II The Reaction Of Iron With Copper (Ii) Chloride Lab Answers This lab will be an attempt to get the highest possible percent yield in performing a single. To determine the number of moles of copper produced, the number of iron used up. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. In this experiment, you will prepare copper metal from the reaction of aluminum metal. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Determining the chemical formula of copper chloride hydrate YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. How many moles of copper would be produced. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From cupsoguepictures.com
😀 Copper chloride and iron. Essay about Lab Repot of The Reaction Of Iron With Copper (Ii) Chloride Lab Answers This lab will be an attempt to get the highest possible percent yield in performing a single. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). To determine the number of moles of. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Aluminum + copper II chloride YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of iron used in the reaction of iron and copper (ii) chloride. How many moles of copper would be produced by reacting. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Add approximately 30 ml of the copper (ii) chloride. Take out the. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From studylib.net
aluminumcopperchloridelab (1) The Reaction Of Iron With Copper (Ii) Chloride Lab Answers To determine the number of moles of copper produced, the number of iron used up. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of copper produced in the. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.chegg.com
Solved If 15 grams of copper (II) chloride react with 20 The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Add approximately 30 ml of the copper (ii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. To determine the number of moles of copper produced,. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.chegg.com
Solved Lab 5 Chemical Reactions quiz questions Reaction. I The Reaction Of Iron With Copper (Ii) Chloride Lab Answers To determine the number of moles of copper produced, the number of iron used up. Also to determine the ratio. Add approximately 30 ml of the copper (ii) chloride. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). This lab will be an attempt to get the. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.westlab.com.au
Copper (II) Chloride Dihydrate AR The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Also to determine the ratio. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. To determine the number of moles of copper produced, the number of iron used up. This lab. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. To determine the number of moles of copper produced, the number of iron used up. This lab will be an attempt to get the highest possible percent yield in performing a single. Determine moles of copper. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Iron + Copper (II) Chloride Reaction Lab Intro YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of iron used in the reaction of iron and copper (ii) chloride. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). This lab will be an attempt to get the highest possible percent yield in performing a single. To determine the number of moles of. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.coursehero.com
[Solved] iron filings added to copper(II) sulfate in solution (assume The Reaction Of Iron With Copper (Ii) Chloride Lab Answers How many moles of copper would be produced by reacting. Also to determine the ratio. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. This lab will be an attempt to get the highest possible percent yield in performing a single.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Representing Reactions Aluminum and Copper (II) Chloride YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Also to determine the ratio. Take out the 100 ml beaker. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. To determine the number of moles of. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From mycentennial.sd43.bc.ca
Chemistry 11 Core Competency SelfAssessment Shiva’s Blog The Reaction Of Iron With Copper (Ii) Chloride Lab Answers This lab will be an attempt to get the highest possible percent yield in performing a single. How many moles of copper would be produced by reacting. Take out the 100 ml beaker. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Write the balanced chemical equation for the reaction of iron metal with copper. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From slideplayer.com
Introduction to Chemical Reactions ppt download The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Add approximately 30 ml of the copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. To determine the number of moles of copper produced, the number of iron used up. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate).. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.numerade.com
SOLVED Texts Part III Preparation of Copper(II) Oxide From Copper The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of copper produced in the reaction of iron and copper (ii) chloride. How many moles of copper would be produced by reacting. This lab will be an attempt to get the highest possible percent yield in performing a single. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From mackenzie-chapter.blogspot.com
Aluminum Copper Ii Chloride Reaction Lab Answers 50+ Pages Summary Doc The Reaction Of Iron With Copper (Ii) Chloride Lab Answers In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Add approximately 30 ml of the copper (ii) chloride. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. This. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From newbedev.com
Chemistry Reaction between copper (II)chloride and aluminium foil The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Add approximately 30 ml of the copper (ii) chloride. Take out the 100 ml beaker. Determine moles of iron used in the reaction of iron and copper (ii) chloride. This lab will be an attempt to get the highest possible percent yield in performing a single.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From mackenzie-chapter.blogspot.com
Aluminum Copper Ii Chloride Reaction Lab Answers 50+ Pages Summary Doc The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Add approximately 30 ml of the copper (ii) chloride. How many moles of copper would be produced by reacting. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Take out the 100 ml beaker. Determine moles of iron used in the. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Lab Activity Reaction of Copper Sulphate and Iron, Class 7 Physics The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Take out the 100 ml beaker. How many moles of copper would be produced by reacting. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. This lab. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.chegg.com
Solved You want to react copper (I) chloride with iron The Reaction Of Iron With Copper (Ii) Chloride Lab Answers This lab will be an attempt to get the highest possible percent yield in performing a single. How many moles of copper would be produced by reacting. Take out the 100 ml beaker. Also to determine the ratio. Determine moles of iron used in the reaction of iron and copper (ii) chloride. In this experiment, you will prepare copper metal. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Aluminum and Copper (II) Chloride Reaction YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of iron used in the reaction of iron and copper (ii) chloride. How many moles of copper would be produced by reacting. Also to determine the ratio. To determine the number of moles of copper produced, the number of iron used up. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. In this. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Take out the 100 ml beaker. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. This lab will be an attempt to get the highest possible percent yield in performing a. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.pinterest.com
A single replacement reaction involving aluminum and a solution of The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Also to determine the ratio. Add approximately 30 ml of the copper (ii) chloride. Take out the 100. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From slideplayer.com
Unit 7 Chemical Reactions Conservation of Mass ppt download The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Add approximately 30 ml of the copper (ii) chloride. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). How many moles of copper would be produced by reacting. This lab will be an. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.mahaautomation.com
COPPER (II) CHLORIDE DIHYDRATE The Reaction Of Iron With Copper (Ii) Chloride Lab Answers To determine the number of moles of copper produced, the number of iron used up. Write the balanced chemical equation for the reaction of iron metal with copper (ii) chloride to create solid copper metal and iron (iii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride. Take out the 100 ml beaker. In. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.reddit.com
Question about Copper (II) Chloride r/chemistry The Reaction Of Iron With Copper (Ii) Chloride Lab Answers To determine the number of moles of copper produced, the number of iron used up. In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(ii) sulfate (cupric sulfate). Add approximately 30 ml of the copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.chegg.com
Solved Data Experiment 8; Preparation of Copper(I) Chloride The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of copper produced in the reaction of iron and copper (ii) chloride. To determine the number of moles of copper produced, the number of iron used up. This lab will be an attempt to get the highest possible percent yield in performing a single. Determine moles of iron used in the reaction of iron and copper (ii) chloride.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.chegg.com
Task 3. Empirical formula for copper (II) chloride The Reaction Of Iron With Copper (Ii) Chloride Lab Answers To determine the number of moles of copper produced, the number of iron used up. This lab will be an attempt to get the highest possible percent yield in performing a single. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Determine moles of iron used in the reaction of iron and copper (ii) chloride.. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From clairebearpsp.tumblr.com
Claire's Physical Science Project Aluminum + Copper Chloride The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of iron used in the reaction of iron and copper (ii) chloride. To determine the number of moles of copper produced, the number of iron used up. Add approximately 30 ml of the copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. How many moles of copper would be produced. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From www.youtube.com
Signs of Chemical Change Aluminum & Copper(II) Chloride Lab YouTube The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Take out the 100 ml beaker. To determine the number of moles of copper produced, the number of iron used up. Determine moles of copper produced in the reaction of iron and copper (ii) chloride. Suppose that you have an unlimited supply of copper(ii) chloride to react with iron. This lab will be an attempt to get the highest possible. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental The Reaction Of Iron With Copper (Ii) Chloride Lab Answers Determine moles of iron used in the reaction of iron and copper (ii) chloride. Take out the 100 ml beaker. Write the complete balanced chemical equation for the reaction between copper (ii) chloride solution. How many moles of copper would be produced by reacting. Also to determine the ratio. Suppose that you have an unlimited supply of copper(ii) chloride to. The Reaction Of Iron With Copper (Ii) Chloride Lab Answers.