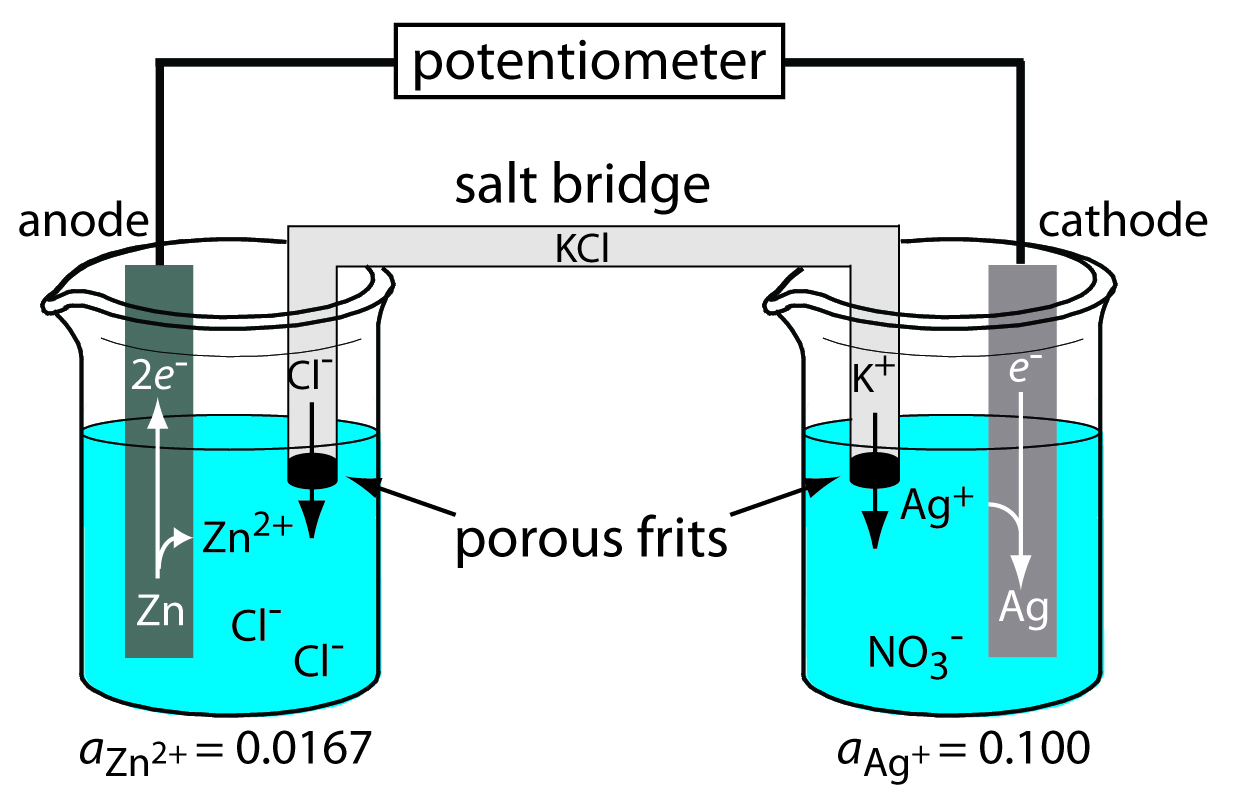
Potentiometer Instrument Chemistry . Because no current—or only a. It is intended to be a primary. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. This module provides an introduction to the measurement technique of potentiometry. The cell is 2 half cells. Measures potential under very low currents. Because no current—or only a negligible. Consist of a reference electrode, indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. In potentiometry we measure the potential of an electrochemical cell under static conditions.
from chem.libretexts.org
It is intended to be a primary. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible. Measures potential under very low currents. This module provides an introduction to the measurement technique of potentiometry. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of.
11.2 Potentiometric Methods Chemistry LibreTexts
Potentiometer Instrument Chemistry Because no current—or only a negligible. The cell is 2 half cells. Measures potential under very low currents. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. It is intended to be a primary. In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. This module provides an introduction to the measurement technique of potentiometry.
From behealthiers.com
Potentiometric analysis methods and their types Potentiometer Instrument Chemistry Because no current—or only a. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Because no current—or only a negligible. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. This module provides an introduction to the measurement technique of potentiometry. Measures potential. Potentiometer Instrument Chemistry.
From www.japson.com
Digital Potentiometer (With inbuilt Stirrer) Measurement Meters for Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Measures potential under very low currents. Consist of a reference electrode, indicator. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. It is intended to be a. Potentiometer Instrument Chemistry.
From www.theengineeringknowledge.com
What is a Potentiometer? Types, Uses, Working Principle & Applications Potentiometer Instrument Chemistry Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. This module provides an introduction to the measurement technique of potentiometry. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. In potentiometry we measure the potential of an electrochemical cell under. Potentiometer Instrument Chemistry.
From www.sciencephoto.com
Potentiometer Stock Image C019/4875 Science Photo Library Potentiometer Instrument Chemistry Measures potential under very low currents. Consist of a reference electrode, indicator. Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Because no current—or only a. Potentiometry is an electrochemical. Potentiometer Instrument Chemistry.
From www.youtube.com
Potentiometer Explained YouTube Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Because no current—or only a negligible. The cell is 2 half cells. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an. Potentiometer Instrument Chemistry.
From www.youtube.com
Applications of potentiometer YouTube Potentiometer Instrument Chemistry In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a. Consist of a reference electrode, indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. It. Potentiometer Instrument Chemistry.
From www.biophlox.com
Buy Digital Potentiometer get price for lab equipment Potentiometer Instrument Chemistry It is intended to be a primary. In potentiometry we measure the potential of an electrochemical cell under static conditions. Consist of a reference electrode, indicator. Because no current—or only a. Measures potential under very low currents. This module provides an introduction to the measurement technique of potentiometry. Potentiometric methods are based upon measurements of the potential of electrochemical cells. Potentiometer Instrument Chemistry.
From www.youtube.com
How does potentiometry work? (With real examples) YouTube Potentiometer Instrument Chemistry This module provides an introduction to the measurement technique of potentiometry. It is intended to be a primary. Consist of a reference electrode, indicator. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under. Potentiometer Instrument Chemistry.
From blog.pishop.co.za
Potentiometer Definition, Types and Working Principle Blog Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only. Potentiometer Instrument Chemistry.
From www.etechnog.com
Potentiometer Diagram, Symbol, and Construction ETechnoG Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only a. Because no current—or only a negligible. Measures potential under very low currents. Potentiometric methods are based upon measurements of. Potentiometer Instrument Chemistry.
From www.indiamart.com
Digital Potentiometer, for Measurement and Test Equipment at best price Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Because no current—or only a negligible. Consist of a reference electrode, indicator. It is intended to be a primary. Because no current—or only a. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the. Potentiometer Instrument Chemistry.
From www.youtube.com
POTENTIOMETER COMPARISON OF E.M.F s (GRADE12) Prakash & Faisal YouTube Potentiometer Instrument Chemistry Measures potential under very low currents. Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. The cell is 2 half cells. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of. Potentiometer Instrument Chemistry.
From www.philipharris.co.uk
1K Potentiometer B8R05958 Philip Harris Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. Consist of a reference electrode, indicator. It is intended to be a primary. Potentiometry is an electrochemical method that measures the electrical potential between two. Potentiometer Instrument Chemistry.
From www.differencebetween.com
Difference Between pH Meter and Potentiometer Compare the Difference Potentiometer Instrument Chemistry In potentiometry we measure the potential of an electrochemical cell under static conditions. It is intended to be a primary. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. In potentiometry we measure the potential of an electrochemical cell under static conditions. Consist of a reference electrode, indicator. Potentiometry is an electrochemical method. Potentiometer Instrument Chemistry.
From www.youtube.com
Potentiometers Basic Introduction YouTube Potentiometer Instrument Chemistry In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible. This module provides an introduction to the measurement technique of potentiometry. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. It is intended to be a primary. The cell is. Potentiometer Instrument Chemistry.
From medilabexports.com
Digital Potentiometer Medilab Exports Consortium Potentiometer Instrument Chemistry Because no current—or only a negligible. This module provides an introduction to the measurement technique of potentiometry. Measures potential under very low currents. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. In. Potentiometer Instrument Chemistry.
From www.youtube.com
Potentiometer and Types of Potentiometer YouTube Potentiometer Instrument Chemistry Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. The cell is 2 half cells. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential. Potentiometer Instrument Chemistry.
From equiptronics.com
DIGITAL POTENTIO METER Equiptronics Potentiometer Instrument Chemistry Because no current—or only a negligible. The cell is 2 half cells. Measures potential under very low currents. In potentiometry we measure the potential of an electrochemical cell under static conditions. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. This module provides an introduction to the. Potentiometer Instrument Chemistry.
From www.exportersindia.com
Metal Laboratory Potentiometer, for Industrial Use, Feature Durable Potentiometer Instrument Chemistry Measures potential under very low currents. Because no current—or only a. Because no current—or only a negligible. The cell is 2 half cells. This module provides an introduction to the measurement technique of potentiometry. In potentiometry we measure the potential of an electrochemical cell under static conditions. It is intended to be a primary. Potentiometry is an electrochemical method that. Potentiometer Instrument Chemistry.
From www.youtube.com
What is a potentiometer. How does a potentiometer work. Potentiometer Potentiometer Instrument Chemistry Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Measures potential under very low currents. It is intended to be a primary. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Consist of a reference electrode, indicator. Because no current—or only a.. Potentiometer Instrument Chemistry.
From www.indiamart.com
Digital Potentiometer at Rs 5000 Chemistry Lab Glassware Instruments Potentiometer Instrument Chemistry In potentiometry we measure the potential of an electrochemical cell under static conditions. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. It is intended to be a primary. This module provides an introduction to the measurement technique of potentiometry.. Potentiometer Instrument Chemistry.
From www.servoinstrument.com
Custom Precision Potentiometers by Servo Instrument Corporation Potentiometer Instrument Chemistry Consist of a reference electrode, indicator. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. The cell is 2 half cells. This module provides an introduction to the measurement technique of. Potentiometer Instrument Chemistry.
From www.linquip.com
Types of Potentiometers Rotary, Linear and Digital Linquip Potentiometer Instrument Chemistry Consist of a reference electrode, indicator. The cell is 2 half cells. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Measures potential under very low currents. Potentiometric methods are based upon measurements of the potential. Potentiometer Instrument Chemistry.
From www.indiamart.com
Laboratory Digital Potentio Meter Device at Rs 4700 Korattur Potentiometer Instrument Chemistry Measures potential under very low currents. The cell is 2 half cells. Because no current—or only a negligible. Consist of a reference electrode, indicator. It is intended to be a primary. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the. Potentiometer Instrument Chemistry.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Potentiometer Instrument Chemistry Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Measures potential under very low currents. In potentiometry we measure the potential of an electrochemical cell under static. Potentiometer Instrument Chemistry.
From koehlerinstrument.com
Automatic Potentiometric Titrator Koehler Instrument Company, Inc. Potentiometer Instrument Chemistry The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Measures potential under very low currents. Because no current—or only a negligible. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. It is intended to be a. Potentiometer Instrument Chemistry.
From www.tubesandmore.com
Potentiometer Alpha, 25K Linear Antique Electronic Supply™ Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Measures potential under very low currents. In potentiometry we measure the potential of an electrochemical cell under static conditions. It is intended to be a primary. In potentiometry we measure the potential of an electrochemical cell under static conditions. Consist of a reference electrode,. Potentiometer Instrument Chemistry.
From www.youtube.com
Potentiometer Instrument Used in Physical Chemistry Part III B. Sc Potentiometer Instrument Chemistry Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. In potentiometry we measure the potential of an electrochemical cell under static conditions. Measures potential under very low currents. Consist of a reference electrode, indicator. Because no current—or only a. The cell is 2 half cells. Potentiometry is an electrochemical. Potentiometer Instrument Chemistry.
From www.servoinstrument.com
Custom Precision Potentiometers by Servo Instrument Corporation Potentiometer Instrument Chemistry Because no current—or only a. Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Measures potential under very low currents. This module provides an introduction to the measurement. Potentiometer Instrument Chemistry.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometer Instrument Chemistry The cell is 2 half cells. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of.. Potentiometer Instrument Chemistry.
From medium.com
Comprehensive Guide to Potentiometers by Jotrinelectronic Medium Potentiometer Instrument Chemistry Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. It is intended to be a primary. Because no current—or only a. In potentiometry we measure the potential of an electrochemical cell under static conditions. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential. Potentiometer Instrument Chemistry.
From www.servoinstrument.com
Custom Precision Potentiometers by Servo Instrument Corporation Potentiometer Instrument Chemistry Measures potential under very low currents. The cell is 2 half cells. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Consist of a reference electrode, indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. It is intended to be a primary. Because no current—or only a negligible.. Potentiometer Instrument Chemistry.
From www.btcinstrument.com
Chemistry Lab Instruments ISI Student Microscope Manufacturer from Ambala Potentiometer Instrument Chemistry The cell is 2 half cells. Measures potential under very low currents. Because no current—or only a negligible. Because no current—or only a. Consist of a reference electrode, indicator. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Potentiometry is an electrochemical method that measures the electrical potential between. Potentiometer Instrument Chemistry.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometer Instrument Chemistry Consist of a reference electrode, indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of. Because no current—or only a. Potentiometer Instrument Chemistry.
From www.youtube.com
Potentiometer for class 12 // Comparison Of EMF Of Two Cells using Potentiometer Instrument Chemistry Because no current—or only a negligible. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Because. Potentiometer Instrument Chemistry.