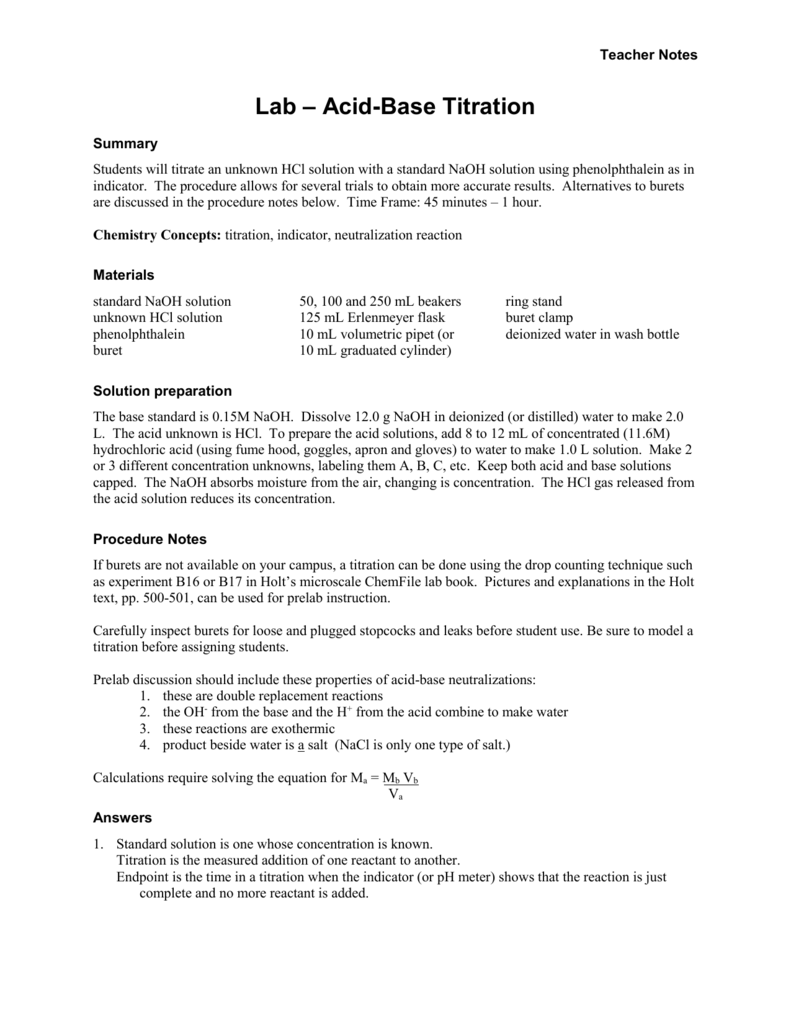
Acid And Base Titration Lab Answers . Study with quizlet and memorize flashcards. To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. The analyte (titrand) is the solution with an. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Calculations b for determination of molecular weight of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; The resulting ph of such.
from mungfali.com
Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Calculations b for determination of molecular weight of an unknown acid. Study with quizlet and memorize flashcards. The analyte (titrand) is the solution with an. Titration is an analytical quantitative technique used to determine the concentration of a solute; The resulting ph of such. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. To use a previously standardized solution to determine the concentration of an unknown acid.
Acid Base Titration Lab
Acid And Base Titration Lab Answers The resulting ph of such. To use a previously standardized solution to determine the concentration of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. The resulting ph of such. The analyte (titrand) is the solution with an. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Study with quizlet and memorize flashcards. Calculations b for determination of molecular weight of an unknown acid.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid And Base Titration Lab Answers The resulting ph of such. The analyte (titrand) is the solution with an. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Titration is an analytical quantitative technique used to determine the concentration of a. Acid And Base Titration Lab Answers.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Study with quizlet and memorize flashcards. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak. Acid And Base Titration Lab Answers.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Acid And Base Titration Lab Answers Calculations b for determination of molecular weight of an unknown acid. The resulting ph of such. To use a previously standardized solution to determine the concentration of an unknown acid. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Study with quizlet. Acid And Base Titration Lab Answers.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a. Acid And Base Titration Lab Answers.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Acid And Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculations b for determination of molecular weight of an unknown acid. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions.. Acid And Base Titration Lab Answers.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react. Acid And Base Titration Lab Answers.
From www.sliderbase.com
Titration Acid And Base Titration Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. Calculations b for determination of molecular weight of an unknown acid. The analyte (titrand) is the solution with an. Study with quizlet and memorize. Acid And Base Titration Lab Answers.
From 2021notubenju.blogspot.com
[11+] Acid Base Review Answers Important Questions Of Acid And Bases Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Precise density of your standardized solution of naoh. Calculations b for determination of. Acid And Base Titration Lab Answers.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Acid And Base Titration Lab Answers The analyte (titrand) is the solution with an. Calculations b for determination of molecular weight of an unknown acid. To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a. Acid And Base Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Acid And Base Titration Lab Answers Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. The resulting ph of such. When exactly equivalent amounts of a strong acid and a strong base are. Acid And Base Titration Lab Answers.
From studyfinder.org
Unveiling Acid Base Titration Pre Lab Answers A Comprehensive Guide Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Precise density of your standardized solution of naoh. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards. Be able to determine the. Acid And Base Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Acid And Base Titration Lab Answers The resulting ph of such. Calculations b for determination of molecular weight of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh.. Acid And Base Titration Lab Answers.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards. To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. When exactly equivalent amounts of a strong acid and a. Acid And Base Titration Lab Answers.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid And Base Titration Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; The resulting ph of such. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Study with quizlet and memorize flashcards. Precise. Acid And Base Titration Lab Answers.
From www.numerade.com
REPORT SHEET EXPERIMENT Titration of Acids and Bases A. Analysis of an Acid And Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; To use a previously standardized solution to determine the concentration of an unknown acid. The analyte (titrand) is the solution with an. Calculations b. Acid And Base Titration Lab Answers.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid And Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine the concentration of a solute; When exactly equivalent amounts of a strong acid and a strong base are mixed together, both. Acid And Base Titration Lab Answers.
From mavink.com
Acid Base Titration Lab Procedure Acid And Base Titration Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. The resulting ph of such. Study with quizlet and memorize flashcards containing terms like to determine. Acid And Base Titration Lab Answers.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Acid And Base Titration Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Calculations b for determination of molecular weight of an unknown acid. The analyte (titrand) is the solution with an.. Acid And Base Titration Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Acid And Base Titration Lab Answers Precise density of your standardized solution of naoh. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of. Acid And Base Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Acid And Base Titration Lab Answers Precise density of your standardized solution of naoh. The analyte (titrand) is the solution with an. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce. Acid And Base Titration Lab Answers.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. The analyte (titrand) is the solution with an. To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. Study with quizlet. Acid And Base Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Study with quizlet and memorize flashcards. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Titration is an analytical quantitative technique used. Acid And Base Titration Lab Answers.
From www.docsity.com
Acid base titration lab answers Docsity Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. The analyte (titrand) is the solution with an. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. The resulting ph of such.. Acid And Base Titration Lab Answers.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Acid And Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculations b for determination of molecular weight of an unknown acid. Precise density of your standardized solution of naoh. The resulting ph of such. When exactly equivalent amounts of a strong acid and a strong base are mixed. Acid And Base Titration Lab Answers.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. The analyte (titrand) is the solution with an. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Titration is an analytical quantitative. Acid And Base Titration Lab Answers.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Calculations b for determination of molecular weight of an unknown acid. Study with quizlet and memorize flashcards. The analyte (titrand) is the solution with an. Titration is an analytical quantitative technique used to. Acid And Base Titration Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 10 A. Acid And Base Titration Lab Answers The resulting ph of such. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. The analyte (titrand) is the. Acid And Base Titration Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Acid And Base Titration Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; The resulting ph of such. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. When exactly equivalent amounts of. Acid And Base Titration Lab Answers.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Acid And Base Titration Lab Answers Calculations b for determination of molecular weight of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; The resulting ph of such. To use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the. Acid And Base Titration Lab Answers.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Acid And Base Titration Lab Answers Calculations b for determination of molecular weight of an unknown acid. The analyte (titrand) is the solution with an. When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. To use a previously standardized solution to determine the concentration of an unknown acid.. Acid And Base Titration Lab Answers.
From joixjswcw.blob.core.windows.net
Titration Of Acid Base Experiment at Joanne Rivera blog Acid And Base Titration Lab Answers The analyte (titrand) is the solution with an. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Study with quizlet and memorize flashcards. Titration is an analytical quantitative technique used to determine the concentration of a solute; Precise density of your standardized solution of naoh. Calculations b for determination. Acid And Base Titration Lab Answers.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Acid And Base Titration Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. When exactly equivalent amounts of a. Acid And Base Titration Lab Answers.
From yazminewadiaz.blogspot.com
Acid Base Titration Lab Report Answers YazminewaDiaz Acid And Base Titration Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Study with quizlet and memorize flashcards. Calculations b for determination of molecular weight of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; When exactly equivalent amounts of a strong. Acid And Base Titration Lab Answers.
From exymuypdr.blob.core.windows.net
Titration Acid Base Experiment at Robert Prescott blog Acid And Base Titration Lab Answers When exactly equivalent amounts of a strong acid and a strong base are mixed together, both species react essentially completely to produce only water and spectator ions. Titration is an analytical quantitative technique used to determine the concentration of a solute; To use a previously standardized solution to determine the concentration of an unknown acid. The resulting ph of such.. Acid And Base Titration Lab Answers.
From studylib.net
Acid/Base Titration Lab Acid And Base Titration Lab Answers Study with quizlet and memorize flashcards containing terms like to determine the mass percent of acetic acid in a vinegar. Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards. The analyte (titrand) is the solution with an. Precise density of your standardized solution of naoh. To use a previously. Acid And Base Titration Lab Answers.