X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 . The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Two lone pairs and four. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: In which of the following pairs, the two species are isostructural: Draw structures of xef6, xeo3, xeof4, xef2. The structure of x e f 4 is shown below. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The electron pair geometry is octahedral and molecular geometry is square. Draw structure and name the shape of bromine trifluoride. Play quiz games with your school.
from www.doubtnut.com
Draw structure and name the shape of bromine trifluoride. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: The electron pair geometry is octahedral and molecular geometry is square. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Two lone pairs and four. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structures of xef6, xeo3, xeof4, xef2. The structure of x e f 4 is shown below. In which of the following pairs, the two species are isostructural: Play quiz games with your school.
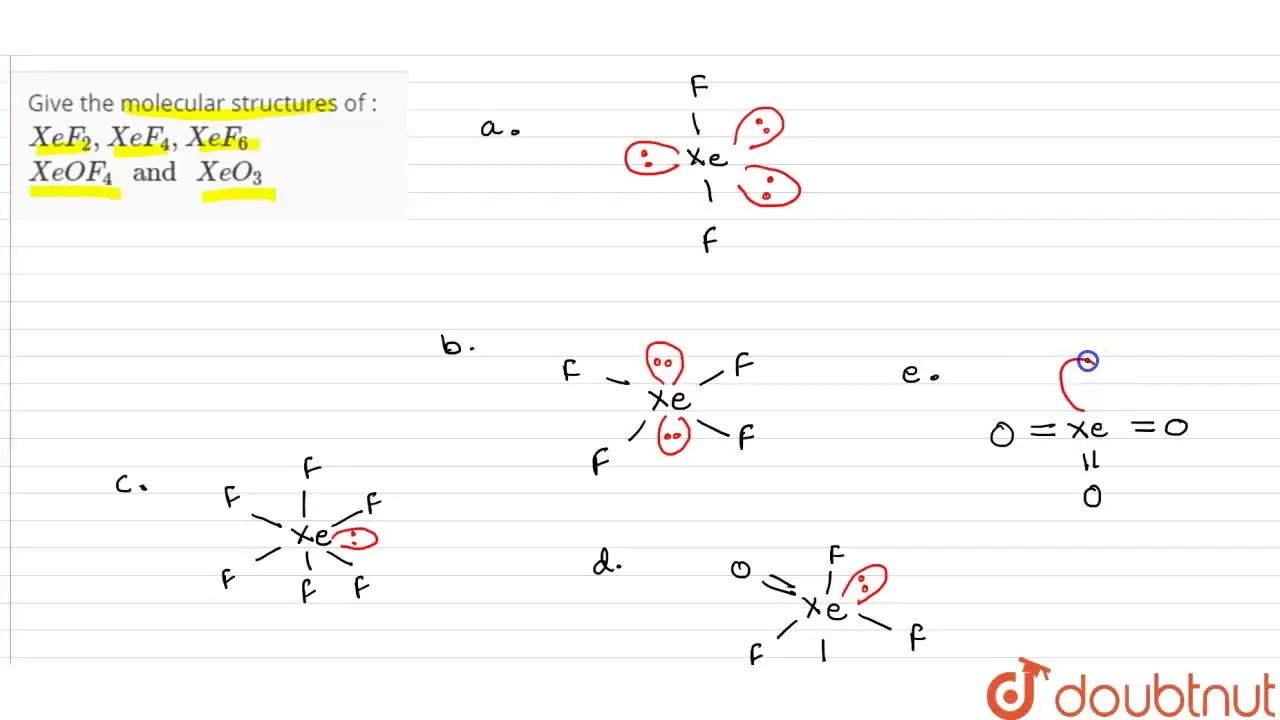
Give the molecular structures of XeF(2),XeF(4),XeF(6) XeOF(4)
X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The central xe atom has 2 lone pairs and 4 bond pairs of electrons. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: The electron pair geometry is octahedral and molecular geometry is square. Draw structures of xef6, xeo3, xeof4, xef2. In which of the following pairs, the two species are isostructural: The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Two lone pairs and four. Draw structure and name the shape of bromine trifluoride. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Play quiz games with your school.
From byjus.com
The shape/structure of [XeF5] and XeO3F2, respectively, are X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The electron pair geometry is octahedral and molecular geometry is square. Draw structure and name the shape of bromine trifluoride. The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at the central. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From chemistryakx.blogspot.com
XeF2 structure, (All knowledge about XeF2) X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Two lone pairs and four. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: The electron pair. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Lewis Structure Of Xef6 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The central xe atom has 2 lone pairs and 4 bond pairs of electrons. In which of the following pairs, the two species are isostructural: What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Draw structure and name the shape of bromine trifluoride. Two lone. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.toppr.com
Xenon hexafluoride on partial hydrolysis produces compounds 'X' and 'Y X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Draw structures of xef6, xeo3, xeof4, xef2. Two lone pairs and four. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Play quiz games with your school. In which of the following pairs, the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.toppr.com
Xenon hexafluoride on partial hydrolysis produces compounds 'X' and 'Y X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Play quiz games with your school. The electron pair geometry is octahedral and molecular geometry is square. The. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From techiescientist.com
XeOF4 Lewis Structure, Geometry, Hybridization, and Polarity X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Draw structures of xef6, xeo3, xeof4, xef2. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structure and name the shape of bromine trifluoride. Problem 4. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Draw The Lewis Structure For The Following Molecule Xef4 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structures of xef6, xeo3, xeof4, xef2. Play quiz games with your school. The electron pair geometry is octahedral and molecular geometry is square. Draw structure and name the shape of bromine trifluoride. What will be the formula of an oxide of iodine (atomic mass =. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.vedantu.com
Draw the structure of xenon hexafluoride (Xe{{F}_{6}}) molecule and X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Play quiz games with your school. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structures of xef6, xeo3, xeof4, xef2. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: In which of the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From byjus.com
49.Do XeF_4 and XeOF_2 both process planar structure X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Draw structure and name the shape of bromine trifluoride. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Play quiz games with your school. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The electron pair geometry is octahedral and molecular geometry is. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From lambdageeks.com
XeF4 Lewis Structure Drawing easy steps,Hybridization,shape X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The central xe atom has 2 lone pairs and 4 bond pairs of electrons. The structure of x e f 4 is shown below. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Draw structures of xef6, xeo3, xeof4, xef2. Problem 4 draw the lewis structures and give the hybridization at the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From quizlet.com
\mathrm{XeOF}_4 has one of the more interesting structures Quizlet X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Draw structure and name the shape of bromine trifluoride. Two lone pairs and four. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom:. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From bilag.xxl.no
Draw The Lewis Structure For Xef2 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The structure of x e f 4 is shown below. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structures of xef6, xeo3, xeof4, xef2. The electron pair geometry is octahedral and molecular geometry is square. In which of the following pairs, the two species are isostructural: Problem 4 draw the lewis structures and. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From brainly.in
draw the structure of XeF^4 ? Brainly.in X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Play quiz games with your school. In which of the following pairs, the two species are isostructural: The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Xef2 Lewis Dot Structure X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Play quiz games with your school. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Two lone pairs and four. The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.youtube.com
Draw the shape of following molecules according to VSEPR theory,XeO_(3 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. Play quiz games with your school. In which of the following pairs, the two species are isostructural: Draw structure and name the shape of bromine trifluoride. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Problem 4 draw the lewis structures. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From draweasy9.blogspot.com
Xef2 Lewis Structure Lone Pairs Drawing Easy X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The structure of x e f 4 is shown below. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Draw structures of xef6, xeo3, xeof4, xef2. The electron pair geometry is octahedral and molecular geometry is square. Draw. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.youtube.com
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 In which of the following pairs, the two species are isostructural: Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Play quiz games with your school. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Lewis Structure Xef6 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Draw structure and name the shape of bromine trifluoride. Draw structures of xef6, xeo3, xeof4,. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.doubtnut.com
Give the molecular structures of XeF(2),XeF(4),XeF(6) XeOF(4) X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The electron pair geometry is octahedral and molecular geometry is square. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. The structure of x e f 4 is shown below. Two lone pairs and four. Problem 4 draw. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From byjus.com
Draw the structure of the XeF2 molecule indicating the lone pairs. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Draw structures of xef6, xeo3, xeof4, xef2. The electron pair geometry is octahedral and molecular geometry is square. Draw structure and name the shape of bromine trifluoride. The structure of x e f 4 is shown below. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: What will be the formula of. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.doubtnut.com
Draw the molecule structures of XeF(2),XeF(4) and XeO(2)F(2) indicatin X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Play quiz games with your school. In which of the following pairs, the two species are isostructural: Draw structure and name the shape of bromine trifluoride. Problem 4 draw the lewis structures and give the hybridization. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Xeof2 Molecular Geometry X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? In which of the following pairs, the two species are isostructural: The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Play quiz games with your school. The electron pair geometry is. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From draweasy8.blogspot.com
Structure De Lewis De Xef2 Draw Easy X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Draw structure and name the shape of bromine trifluoride. The electron pair geometry is octahedral and molecular geometry is square. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Two lone pairs and four. Draw structures of xef6, xeo3, xeof4, xef2. What will be the formula of an oxide of iodine (atomic. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.toppr.com
How are Xeoz and XOF 4 prepared? Give the structures. ) Preparation X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Draw structures of xef6, xeo3, xeof4, xef2. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Problem 4 draw the lewis structures and give the hybridization at the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.researchgate.net
Ballandstick representation of the Xray structure of XeO3 retrieved X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 In which of the following pairs, the two species are isostructural: Draw structures of xef6, xeo3, xeof4, xef2. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. The structure of x e f 4 is shown below. The electron pair geometry is octahedral and molecular geometry is square. What will be the formula of an. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.toppr.com
2.91 Which of the following molecules has a square pyramidal structure X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Play quiz games with your school. Two lone pairs and four. The electron pair geometry is octahedral and molecular geometry is square. Draw structure and name the shape of bromine trifluoride. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Problem 4 draw the lewis structures and give the hybridization at the central atom of. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From chemistryakx.blogspot.com
XeF2 structure, (All knowledge about XeF2) X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Two lone pairs and four. The structure of x e f 4 is shown below. Draw structures of xef6, xeo3, xeof4, xef2. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.youtube.com
Lewis Structure of XeF2 YouTube X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Two lone pairs and four. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Draw structure and name the shape of bromine trifluoride. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: The central xe atom has. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.doubtnut.com
Draw the structure of the following (i) XeF(2) (ii) BrF(3) X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Draw structures of xef6, xeo3, xeof4, xef2. The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Two lone pairs and four. In which of. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.doubtnut.com
(a) Draw the molecular structure of following compounds (i) XeF(6) X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: In which of the following pairs, the two species are isostructural: Draw structures of xef6, xeo3, xeof4, xef2. Play quiz games with your school. The structure of x e f 4 is shown below. Problem 4 draw the lewis structures and give the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From www.doubtnut.com
Draw the molecular structures of XeF(2), XeF(4) and XeO(2)F(2), indica X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 In which of the following pairs, the two species are isostructural: Draw structure and name the shape of bromine trifluoride. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Play quiz. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From byjus.com
The shape/structure of [XeF5] and XeO3F2, respectively, are X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The structure of x e f 4 is shown below. Two lone pairs and four. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Draw structures of xef6, xeo3, xeof4, xef2. In which of the following pairs, the. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From upload.independent.com
Draw The Lewis Structure For Xef2 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 Draw structures of xef6, xeo3, xeof4, xef2. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Two lone pairs and four. What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? Problem 4 draw the lewis structures and give the hybridization. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From ar.inspiredpencil.com
Molecular Geometry Of Xef2 X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen? The central xe atom has 2 lone pairs and 4 bond pairs of electrons. The lewis structure of xef 4 indicates six regions of high electron density around the xenon atom: Two lone pairs and four.. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.
From lambdageeks.com
XeF4 Lewis Structure Drawing easy steps,Hybridization,shape X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2 The electron pair geometry is octahedral and molecular geometry is square. The central xe atom has 2 lone pairs and 4 bond pairs of electrons. Two lone pairs and four. Draw structures of xef6, xeo3, xeof4, xef2. Problem 4 draw the lewis structures and give the hybridization at the central atom of the following compounds: Play quiz games with your. X Draw Structures Of Xef 6 Xeo 3 Xeof 4 Xef2.