Bromide Anion Polar Or Nonpolar . every sufficiently asymmetric molecule will be polar, but some more than others. explanation of dipole moments in molecules and their significance. Molecules as a whole can also be described as. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. The polarity of molecules is related to the polarity of. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved.
from axispharm.com
If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. The polarity of molecules is related to the polarity of. Molecules as a whole can also be described as. every sufficiently asymmetric molecule will be polar, but some more than others. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. explanation of dipole moments in molecules and their significance. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar.
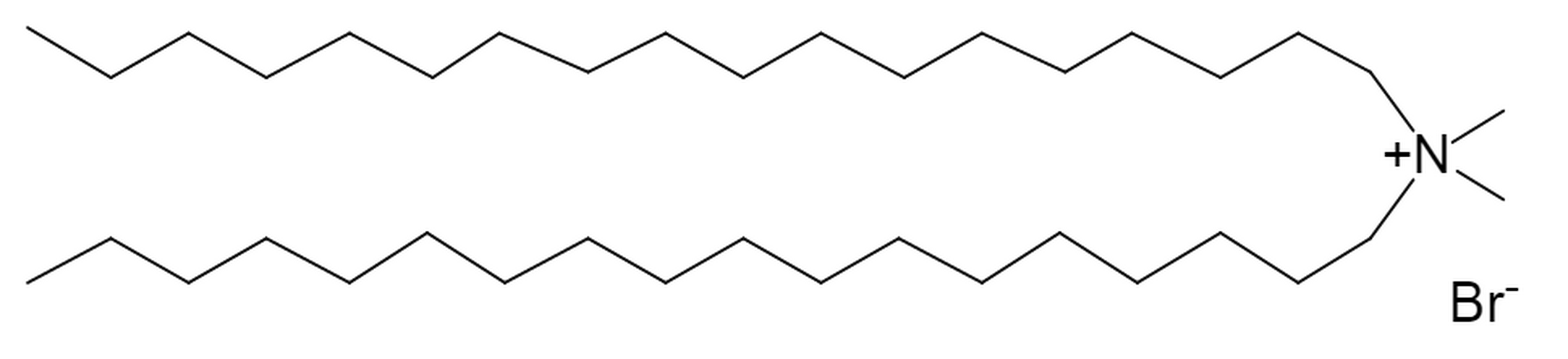
Dimethyldioctadecylammonium bromide AxisPharm
Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. The polarity of molecules is related to the polarity of. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. every sufficiently asymmetric molecule will be polar, but some more than others. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. Molecules as a whole can also be described as. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. explanation of dipole moments in molecules and their significance.
From www.youtube.com
Is HBr Polar or Nonpolar? (Hydrogen bromide) YouTube Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. every sufficiently asymmetric molecule will be polar, but some more than others. Molecules as a whole can also be described as. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. explanation. Bromide Anion Polar Or Nonpolar.
From www.sigmaaldrich.co.th
THIAZOLYL BLUE TETRAZOLIU M BROMIDE, 98 Merck Life Sciences Thailand Bromide Anion Polar Or Nonpolar The polarity of molecules is related to the polarity of. Molecules as a whole can also be described as. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. explanation of dipole moments in molecules. Bromide Anion Polar Or Nonpolar.
From www.youtube.com
Lewis Structure of CsBr, caesium bromide YouTube Bromide Anion Polar Or Nonpolar every sufficiently asymmetric molecule will be polar, but some more than others. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. The polarity of molecules is related to the polarity of. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.. Bromide Anion Polar Or Nonpolar.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. explanation of dipole moments in molecules and their significance. every sufficiently asymmetric molecule will be polar, but some. Bromide Anion Polar Or Nonpolar.
From www.nanochemazone.com
1Dodecyl3methylimidazolium Bromide Low Price 45 High Purity Bromide Anion Polar Or Nonpolar covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The polarity of molecules is related to the polarity of. every sufficiently asymmetric molecule will be polar, but some more than others. If the electronegativity. Bromide Anion Polar Or Nonpolar.
From axispharm.com
Dimethyldioctadecylammonium bromide AxisPharm Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. The polarity. Bromide Anion Polar Or Nonpolar.
From www.nanochemazone.com
1Butyl1methylpyrrolidinium Bromide Low Price 40 High Purity Bromide Anion Polar Or Nonpolar covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Molecules as a whole can also be described as. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of. Bromide Anion Polar Or Nonpolar.
From www.mcguff.com
Ipratropium Bromide, 0.02, Inhalation Solution, 2.5mL, 25 Vials/Tray Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. The polarity of molecules is related to. Bromide Anion Polar Or Nonpolar.
From www.pinterest.dk
Science Coverage Is HBr Polar or Nonpolar? Molecular Geometry Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. The polarity of molecules is related to the polarity of. If the electronegativity difference (usually called δen) is less than. Bromide Anion Polar Or Nonpolar.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Anion Polar Or Nonpolar The polarity of molecules is related to the polarity of. explanation of dipole moments in molecules and their significance. every sufficiently asymmetric molecule will be polar, but some more than others. Molecules as a whole can also be described as. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of. Bromide Anion Polar Or Nonpolar.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Anion Polar Or Nonpolar If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. every sufficiently asymmetric molecule will be polar, but some more than others. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the bond polarity between two atoms can be estimated. Bromide Anion Polar Or Nonpolar.
From fyodahncv.blob.core.windows.net
Magnesium Bromide Molecular Formula at Francis Rogers blog Bromide Anion Polar Or Nonpolar explanation of dipole moments in molecules and their significance. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. Molecules as a whole can also be described as. whether a bond. Bromide Anion Polar Or Nonpolar.
From www.youtube.com
Is BrCl3 Polar or Nonpolar (Bromine Trichloride) YouTube Bromide Anion Polar Or Nonpolar the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. explanation of dipole moments in molecules and their significance. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. Molecules as a whole can also be described as. whether a bond is nonpolar. Bromide Anion Polar Or Nonpolar.
From www.mdpi.com
Molecules Free FullText DessMartin PeriodinaneMediated Oxidative Bromide Anion Polar Or Nonpolar every sufficiently asymmetric molecule will be polar, but some more than others. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. explanation of dipole moments in molecules and their significance. whether a bond is nonpolar or polar covalent is determined by a property of the bonding. Bromide Anion Polar Or Nonpolar.
From www.indiamart.com
N Pentyl Bromide at best price in Mumbai ID 8331150097 Bromide Anion Polar Or Nonpolar The polarity of molecules is related to the polarity of. explanation of dipole moments in molecules and their significance. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. the bond. Bromide Anion Polar Or Nonpolar.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Anion Polar Or Nonpolar describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. explanation of dipole moments in molecules and their significance. the bond polarity between two atoms can be estimated. Bromide Anion Polar Or Nonpolar.
From hxesxnvpp.blob.core.windows.net
Potassium Bromide Compound Name at Kathryn Hudson blog Bromide Anion Polar Or Nonpolar describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. The polarity of molecules is related to the polarity of. Molecules as a whole can also be described as. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. . Bromide Anion Polar Or Nonpolar.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. Molecules as a whole can also be described as. The polarity of molecules is related to the polarity of. . Bromide Anion Polar Or Nonpolar.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Bromide Anion Polar Or Nonpolar explanation of dipole moments in molecules and their significance. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. If the electronegativity difference (usually called δen) is less than 0.5, then. Bromide Anion Polar Or Nonpolar.
From www.numerade.com
SOLVED Need help on atoms and molecules. Choose the letter that best Bromide Anion Polar Or Nonpolar covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. the bond polarity between two atoms. Bromide Anion Polar Or Nonpolar.
From www.mcguff.com
Ipratropium Bromide, 0.02, Inhalation Solution, 2.5mL, 25 Vials/Tray Bromide Anion Polar Or Nonpolar every sufficiently asymmetric molecule will be polar, but some more than others. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. Molecules as a whole can also be described as. whether a. Bromide Anion Polar Or Nonpolar.
From cartoondealer.com
Bromide Cartoons, Illustrations & Vector Stock Images 164 Pictures to Bromide Anion Polar Or Nonpolar the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. whether a bond is. Bromide Anion Polar Or Nonpolar.
From www.numerade.com
SOLVED Need help on atoms and molecules. Choose the letter that best Bromide Anion Polar Or Nonpolar Molecules as a whole can also be described as. The polarity of molecules is related to the polarity of. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. every sufficiently asymmetric molecule will be polar, but some more than others. explanation of dipole moments in molecules and. Bromide Anion Polar Or Nonpolar.
From www.numerade.com
SOLVED Predict the products of the following reactions.(a) allyl Bromide Anion Polar Or Nonpolar whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. the bond polarity between two atoms. Bromide Anion Polar Or Nonpolar.
From sinoleadbio.en.made-in-china.com
Rocuronium Bromide Injection 5ml 50mg. 5ampoules/Box China Bromide Anion Polar Or Nonpolar The polarity of molecules is related to the polarity of. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. explanation of dipole moments in molecules and their significance. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. every sufficiently asymmetric. Bromide Anion Polar Or Nonpolar.
From techiescientist.com
Is Br2 Polar or Nonpolar? Techiescientist Bromide Anion Polar Or Nonpolar The polarity of molecules is related to the polarity of. Molecules as a whole can also be described as. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. whether a. Bromide Anion Polar Or Nonpolar.
From brainly.com
Consider the reaction of an alkyl bromide and hydroxide ion. OH Draw Bromide Anion Polar Or Nonpolar covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Molecules as a whole can also be described as. the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The polarity of molecules is related to the polarity of. every sufficiently asymmetric molecule will be. Bromide Anion Polar Or Nonpolar.
From simp-link.com
Difference between polar and nonpolar examples Bromide Anion Polar Or Nonpolar describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. The polarity of molecules is related to the polarity of. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Molecules as a whole can also be described as. . Bromide Anion Polar Or Nonpolar.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Bromide Anion Polar Or Nonpolar covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. If the electronegativity difference. Bromide Anion Polar Or Nonpolar.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Bromide Anion Polar Or Nonpolar the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. every. Bromide Anion Polar Or Nonpolar.
From www.youtube.com
Is HBr Polar or Nonpolar? (Hydrogen Bromide) YouTube Bromide Anion Polar Or Nonpolar the bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Molecules as a whole can also be described as. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. If the electronegativity difference (usually called δen) is less than 0.5, then the. Bromide Anion Polar Or Nonpolar.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Anion Polar Or Nonpolar every sufficiently asymmetric molecule will be polar, but some more than others. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. The polarity of molecules is related to the polarity of. the bond polarity between two atoms can be estimated if you know the electronegativity of both. Bromide Anion Polar Or Nonpolar.
From www.meritnation.com
7 What happens when ethyl bromide treated with i)KOH aq ii)AgCN iii)NH3 Bromide Anion Polar Or Nonpolar Molecules as a whole can also be described as. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. describe how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar. explanation of dipole moments in molecules and their significance. . Bromide Anion Polar Or Nonpolar.
From www.coursehero.com
[Solved] . 7. A student was trying to figure out whether isopropyl Bromide Anion Polar Or Nonpolar explanation of dipole moments in molecules and their significance. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. The polarity of molecules is related to the polarity of. Molecules as a whole. Bromide Anion Polar Or Nonpolar.
From www.dreamstime.com
3D Image of Rocuronium Bromide Skeletal Formula Stock Illustration Bromide Anion Polar Or Nonpolar If the electronegativity difference (usually called δen) is less than 0.5, then the bond is nonpolar covalent. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. every sufficiently asymmetric molecule will be. Bromide Anion Polar Or Nonpolar.