Drying Chemistry . Always use an erlenmeyer flask, not a beaker. Excluding the partial drying of. Various commonly used organic solvents were dried with several different drying agents. There are many instances when it is necessary to remove traces of water from a solution or liquid. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. If a second layer (water) is seen in the flask, remove it by pipette. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. One common example is the drying of an organic layer after a solvent extraction. Drying agents are used to remove trace amounts of water from an organic solution.
from www.mdpi.com
Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Excluding the partial drying of. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Always use an erlenmeyer flask, not a beaker. There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying agents are used to remove trace amounts of water from an organic solution. One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying agents. If a second layer (water) is seen in the flask, remove it by pipette. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media.
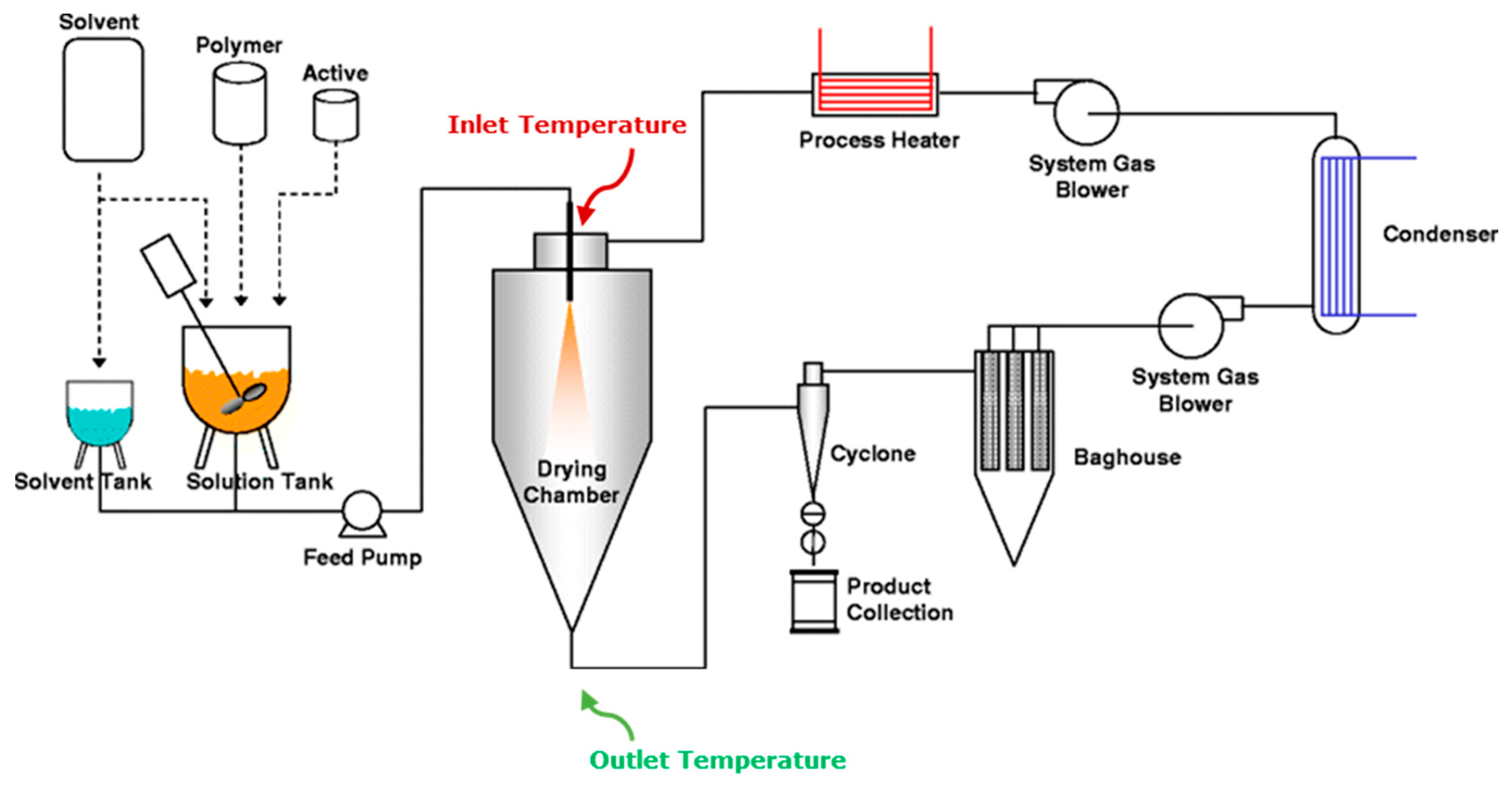
Processes Free FullText Spray Drying for the Preparation of
Drying Chemistry Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Various commonly used organic solvents were dried with several different drying agents. Always use an erlenmeyer flask, not a beaker. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. One common example is the drying of an organic layer after a solvent extraction. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Excluding the partial drying of. There are many instances when it is necessary to remove traces of water from a solution or liquid. If a second layer (water) is seen in the flask, remove it by pipette. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Drying agents are used to remove trace amounts of water from an organic solution.
From phys.org
Spraydrying to produce new materials in industrial applications Drying Chemistry One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying agents. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. If a second layer (water) is seen in the flask, remove it by. Drying Chemistry.
From www.youtube.com
Active Freeze Drying of pharmaceuticals YouTube Drying Chemistry Always use an erlenmeyer flask, not a beaker. Various commonly used organic solvents were dried with several different drying agents. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. One common example is the. Drying Chemistry.
From www.vettertec.com
Spray Dryers VETTERTEC PASSION FOR DRYING Drying Chemistry Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. One common example is the drying of an organic layer after a solvent extraction. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. If a second layer (water) is seen in the flask, remove. Drying Chemistry.
From www.sciencemadness.org
FileDrying Agents Chart.png Sciencemadness Wiki Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. If a second layer (water) is seen in the flask, remove it by pipette. Various commonly used organic solvents were dried with several different drying agents. Drying involves the final removal of relatively small amounts of water, or in. Drying Chemistry.
From chemistryhall.com
Types of Chemistry Flasks A Complete Guide Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Excluding the partial drying of. Drying agents are used to remove trace amounts of water from an organic solution. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. There are many instances. Drying Chemistry.
From ian.umces.edu
Drying oven Media Library Integration and Application Network Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Various commonly used organic solvents were dried with several different drying agents. If a second layer (water) is seen in the flask, remove it by pipette. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water. Drying Chemistry.
From www.youtube.com
How to Use the Hot air gun for Drying Moisture sensitive reactions Drying Chemistry Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Various commonly used organic solvents were dried with several different drying agents. There are many instances when it is necessary to remove traces of water from a solution or liquid. If a second layer (water) is seen in the flask, remove. Drying Chemistry.
From www.pinterest.com
Pictures from an organic chemistry laboratory Organic chemistry Drying Chemistry One common example is the drying of an organic layer after a solvent extraction. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Drying agents are used to remove trace amounts of water from an organic solution. Drying involves the final removal of relatively small amounts of water, or in some cases solvent,. Drying Chemistry.
From www.coursehero.com
[Solved] DRYING PROBLEM 2 A rotary dryer dries a crystalline organic Drying Chemistry Drying agents are used to remove trace amounts of water from an organic solution. If a second layer (water) is seen in the flask, remove it by pipette. One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying agents. There are many instances when it. Drying Chemistry.
From www.reddit.com
Reddit Dive into anything Drying Chemistry One common example is the drying of an organic layer after a solvent extraction. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Always use an erlenmeyer flask, not a beaker. There are many instances when it is necessary to remove traces of water from a solution or. Drying Chemistry.
From www.saintyco.com
Fluid Bed Dryer The Ultimate Guide for Importers SaintyCo Drying Chemistry There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Excluding the partial drying of. If a second layer (water) is seen in the flask, remove it by pipette. Always use an erlenmeyer. Drying Chemistry.
From www.slideserve.com
PPT Drying Basics PowerPoint Presentation, free download ID5121093 Drying Chemistry Drying agents are used to remove trace amounts of water from an organic solution. One common example is the drying of an organic layer after a solvent extraction. If a second layer (water) is seen in the flask, remove it by pipette. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a. Drying Chemistry.
From nwgd.nl
Drying in the chemical industry NWGD Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Excluding the partial drying of. Drying agents are used to remove trace amounts of water from an organic solution. Various commonly used organic solvents were dried with several different drying agents. One common example is the drying of an. Drying Chemistry.
From www.heinkel.com
Leading chemical plant relies on efficient drying with COMBER Vacuum Drying Chemistry Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Always use an erlenmeyer flask, not a beaker. One common example is the drying of an organic layer after a solvent extraction. Excluding the partial drying of. Various commonly used organic solvents were dried with several different drying agents. Drying agents. Drying Chemistry.
From karaulidiagnostics.com
Dry Chemistry In Clinical Pathology. Karauli Diagnostics Drying Chemistry One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying agents. Excluding the partial drying of. Drying agents are used to remove trace amounts of water from an organic solution. This chapter reviews the basics of drying, and outlines various aspects related to drying of. Drying Chemistry.
From slidetodoc.com
BIOCHEMISTRY ANALYZERS Colorimeter Spectrophotometer Semi AutoAnalyzer Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Drying agents are used to remove trace amounts of water from an organic solution. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. If a second layer (water) is seen in the. Drying Chemistry.
From www.mdpi.com
Processes Free FullText Spray Drying for the Preparation of Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Drying agents are used to remove trace amounts of water from an organic solution. There are many instances when it is necessary to remove traces of water from a solution or liquid. One common example is the drying of. Drying Chemistry.
From pubs.acs.org
Continuous Supercritical Drying of Aerogel Particles Proof of Concept Drying Chemistry Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Drying agents are used to remove trace amounts of water from an organic solution. There are many instances when it is necessary to remove traces of water from a solution or liquid. If a second layer (water) is seen in the. Drying Chemistry.
From www.mdpi.com
Processes Free FullText Spray FreezeDrying as a Solution to Drying Chemistry Always use an erlenmeyer flask, not a beaker. One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying agents. There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying agents are anhydrous inorganic materials that favorably. Drying Chemistry.
From www.chemicalslearning.com
Chemical Drying Procedure, and Advantages Drying Chemistry Excluding the partial drying of. There are many instances when it is necessary to remove traces of water from a solution or liquid. Various commonly used organic solvents were dried with several different drying agents. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. If a second layer. Drying Chemistry.
From www.chemistry.nat.fau.eu
More than just drying spraydrying as a way towards multifunctional Drying Chemistry This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Drying agents are used to remove trace amounts of water from an organic solution. There are many instances when it is necessary to remove traces of water from a solution or liquid. One common example is the drying of. Drying Chemistry.
From quizguarantors.z4.web.core.windows.net
What Is A Drying Agent In Chemistry Drying Chemistry Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. One common example is the drying of an organic layer after a solvent extraction. Various commonly used organic solvents were dried with several different drying. Drying Chemistry.
From dorsatech.ir
Article Details Drying Chemistry There are many instances when it is necessary to remove traces of water from a solution or liquid. Various commonly used organic solvents were dried with several different drying agents. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. One common example is the drying of an organic layer after. Drying Chemistry.
From en.seamaty.com
Dry Chemistry Testing Principle Accurate, Simple and Fast A Drying Chemistry Drying agents are used to remove trace amounts of water from an organic solution. Always use an erlenmeyer flask, not a beaker. Various commonly used organic solvents were dried with several different drying agents. There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying agents are anhydrous inorganic materials that favorably. Drying Chemistry.
From www.slideshare.net
Chapter 11 Drying Chemistry Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. One common example is the drying of an organic layer after a solvent extraction. There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying involves the final removal of relatively small amounts of water, or. Drying Chemistry.
From www.researchgate.net
Schematic Representation of SprayDrying Technology. Download Drying Chemistry Excluding the partial drying of. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. If a second layer (water) is seen in the flask, remove it by pipette. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. One common example is the drying. Drying Chemistry.
From www.researchgate.net
The brief diagram of the centrifugal spraydrying apparatus. Download Drying Chemistry Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Excluding the partial drying of. There are many instances when it is necessary to remove traces of water from a solution or liquid. Always use an erlenmeyer flask, not a beaker. Various commonly used organic solvents were dried with several different drying agents. This. Drying Chemistry.
From cen.acs.org
Periodicgraphicschemistrydrycleaning Drying Chemistry Always use an erlenmeyer flask, not a beaker. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Various commonly used organic solvents were dried with several different drying agents. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. One common example is the. Drying Chemistry.
From triogenlife.com
ChemCare Dry Chemistry Analyser Triogen Life Drying Chemistry Various commonly used organic solvents were dried with several different drying agents. If a second layer (water) is seen in the flask, remove it by pipette. One common example is the drying of an organic layer after a solvent extraction. Excluding the partial drying of. This chapter reviews the basics of drying, and outlines various aspects related to drying of. Drying Chemistry.
From www.youtube.com
Dry Chemistry, Spotchem EZ, Arkray Inc, Japan YouTube Drying Chemistry Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. There are many instances when it is necessary to remove traces of water from a solution or liquid. Always use an erlenmeyer flask, not a. Drying Chemistry.
From www.mrclab.com
Laboratory OvensUses/Types/Work Drying Chemistry Always use an erlenmeyer flask, not a beaker. Various commonly used organic solvents were dried with several different drying agents. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Excluding the partial drying of. There are many instances when it is necessary to remove traces of water from. Drying Chemistry.
From halomedicals.com
Wondfo Dry Chemistry Analyzer HALOMEDICALS SYSTEMS LIMITED Drying Chemistry One common example is the drying of an organic layer after a solvent extraction. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. This chapter reviews the basics of drying, and outlines various aspects related to drying of solids, the structure of porous media. Drying agents are used to remove trace amounts of. Drying Chemistry.
From www.chemengonline.com
The Benefits Of TwoStage Drying Chemical Engineering Page 1 Drying Chemistry Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Excluding the partial drying of. One common example is the drying of an organic layer after a solvent extraction. Drying agents are used to remove. Drying Chemistry.
From achs-prod.acs.org
Nanocellulose Dewatering and Drying Current State and Future Drying Chemistry There are many instances when it is necessary to remove traces of water from a solution or liquid. Excluding the partial drying of. Drying agents are anhydrous inorganic materials that favorably form hydrates, which incorporate water molecules into their. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. This chapter. Drying Chemistry.
From studylib.net
Freezedrying Chemistry Central Journal Drying Chemistry Various commonly used organic solvents were dried with several different drying agents. There are many instances when it is necessary to remove traces of water from a solution or liquid. Drying involves the final removal of relatively small amounts of water, or in some cases solvent, from a material. This chapter reviews the basics of drying, and outlines various aspects. Drying Chemistry.