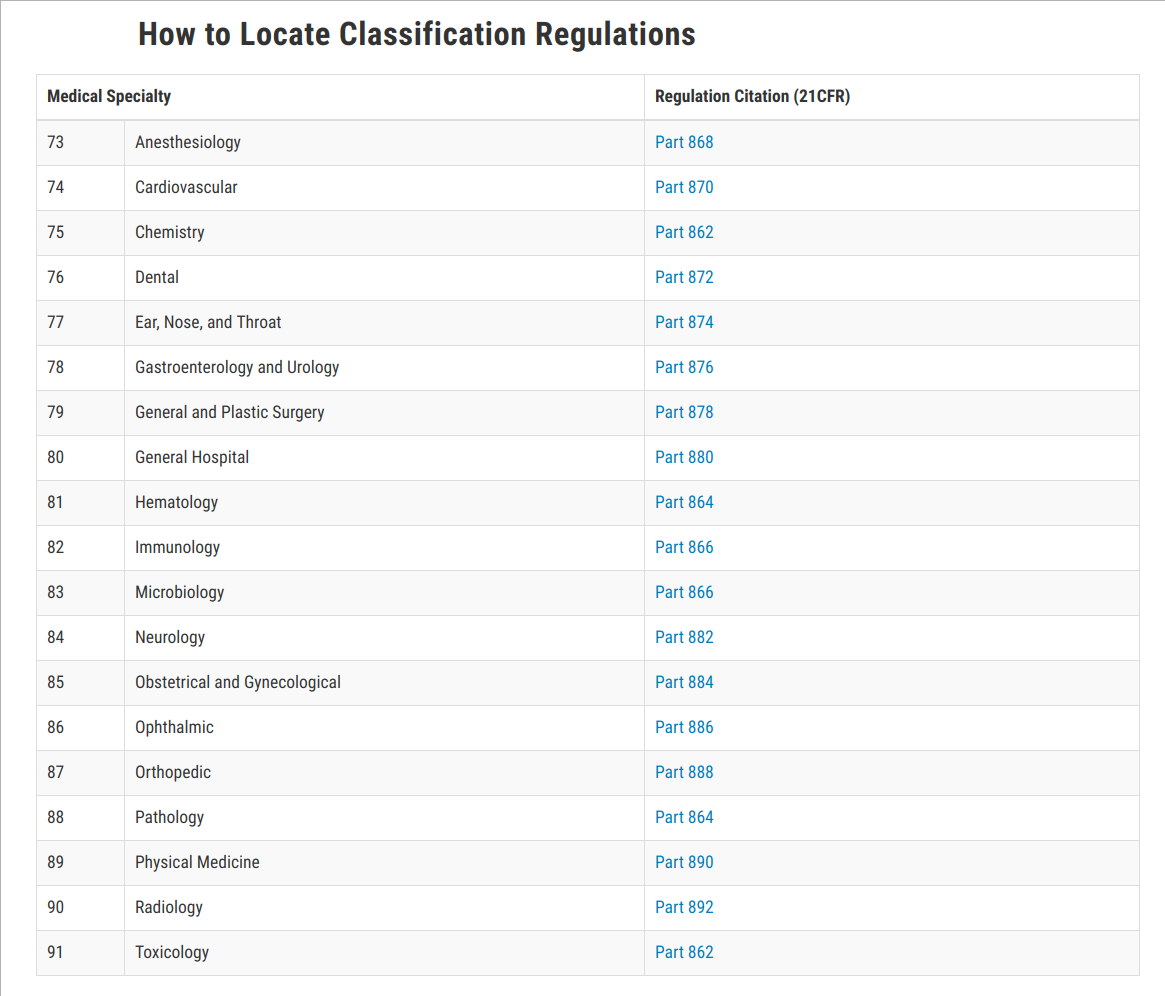
Medical Device Class List . The fda classifies medical devices based on their risk to patient safety. Either class i, ii or iii, depending on its risk,. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Class ii are the second most common class for medical. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the.
from www.qualio.com
Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Either class i, ii or iii, depending on its risk,. The fda classifies medical devices based on their risk to patient safety. Class ii are the second most common class for medical.
Does an FDA Class 1 Medical Device List Exist?
Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Either class i, ii or iii, depending on its risk,. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The fda classifies medical devices based on their risk to patient safety. Class ii are the second most common class for medical. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and.
From www.ce-marking.com
Guide on Class IIb MDD Medical Devices CE marking (mark) & European Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Either class i, ii or iii, depending on its risk,. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Any medical device approved. Medical Device Class List.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Class List Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Most medical devices can be. Medical Device Class List.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Any medical. Medical Device Class List.
From www.regdesk.co
FDA Exemption for Class II Medical Devices RegDesk Medical Device Class List Class ii are the second most common class for medical. Either class i, ii or iii, depending on its risk,. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. For class iii devices, a. Medical Device Class List.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Class List Either class i, ii or iii, depending on its risk,. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: For class iii devices, a premarket approval application (pma) will be required unless your device is a. Medical Device Class List.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Class ii are the second most common class for medical. The fda classifies medical devices based on their. Medical Device Class List.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Class ii are the second most common class for medical. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Either class i,. Medical Device Class List.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Class List Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The fda classifies medical devices. Medical Device Class List.
From chinameddevice.com
CFDA New Medical Device Classification Catalogue Effective August 1st Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Class ii are the second most common class for medical. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii, depending on. Medical Device Class List.
From www.volersystems.com
Are You Making a Medical Device? Voler Systems Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Most medical devices can be classified by finding the matching description of the device in title 21 of the. Medical Device Class List.
From www.youtube.com
Medical Device Classes Explained A Beginner's Guide YouTube Medical Device Class List Either class i, ii or iii, depending on its risk,. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. The fda classifies medical devices based on their risk to patient safety. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts.. Medical Device Class List.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Class List Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Class ii are the second most common class for medical. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. For class iii devices, a. Medical Device Class List.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Class List Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Class ii are the second most common class for medical. Any medical device approved by the fda center for devices and radiological. Medical Device Class List.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Either class i, ii or iii, depending on its risk,. Examples of fda class i medical devices. Medical Device Class List.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Class ii are the second most common class for medical. Either class i, ii. Medical Device Class List.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s.,. Medical Device Class List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. The fda classifies medical devices. Medical Device Class List.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Class ii are the second most common class for medical. Examples of fda class i medical devices. Medical Device Class List.
From www.qualio.com
Does an FDA Class 1 Medical Device List Exist? Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Class ii are the second most common class for medical. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts.. Medical Device Class List.
From www.evidera.com
White Paper The Growing Need for RealWorld Evidence in Medical Medical Device Class List Either class i, ii or iii, depending on its risk,. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Class ii are the second most common class for medical. In the u.s., the fda defines a class ii medical device as a device that. Medical Device Class List.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples. Medical Device Class List.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Class List In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Either class i, ii or iii, depending on its risk,. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. The fda classifies medical devices based on. Medical Device Class List.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Device Class List The fda classifies medical devices based on their risk to patient safety. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Most medical devices can be. Medical Device Class List.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. The fda classifies medical devices based on their risk to patient safety. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii, depending. Medical Device Class List.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Class List Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: The fda classifies medical devices based on their risk to patient safety. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Class ii are. Medical Device Class List.
From vem-medical.com
Medical Device Manufacturing Medical Device Class List The fda classifies medical devices based on their risk to patient safety. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. In the u.s., the fda defines a. Medical Device Class List.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Class List The fda classifies medical devices based on their risk to patient safety. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Class ii are the second. Medical Device Class List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Class List Either class i, ii or iii, depending on its risk,. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Class ii are the second most common class. Medical Device Class List.
From mavink.com
Fda Medical Device Classification Chart Medical Device Class List Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: Either class i, ii or iii, depending on its risk,. In the u.s., the fda defines a class ii medical device as a device that presents moderate. Medical Device Class List.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? Medical Device Class List Either class i, ii or iii, depending on its risk,. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. The fda classifies medical devices based on their risk to patient safety. Any medical device approved by the fda center for devices and radiological health. Medical Device Class List.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class List Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Any. Medical Device Class List.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Class List For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Examples of fda class i medical devices include tongue depressors, manual stethoscopes, and. Most medical devices can be classified by finding the matching description of the device in title 21 of the code of federal regulations (cfr), parts. Class. Medical Device Class List.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Class List Class ii are the second most common class for medical. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Either class i, ii or iii, depending on. Medical Device Class List.
From sunstonepilot.com
Introduction to Medical Device Development Sunstone Pilot, Inc. Medical Device Class List The fda classifies medical devices based on their risk to patient safety. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s., the fda defines a. Medical Device Class List.
From www.researchgate.net
Medical Device Classification System Download Table Medical Device Class List Either class i, ii or iii, depending on its risk,. Any medical device approved by the fda center for devices and radiological health is classified into one of three classes: In the u.s., the fda defines a class ii medical device as a device that presents moderate potential harm to the individual. Examples of fda class i medical devices include. Medical Device Class List.