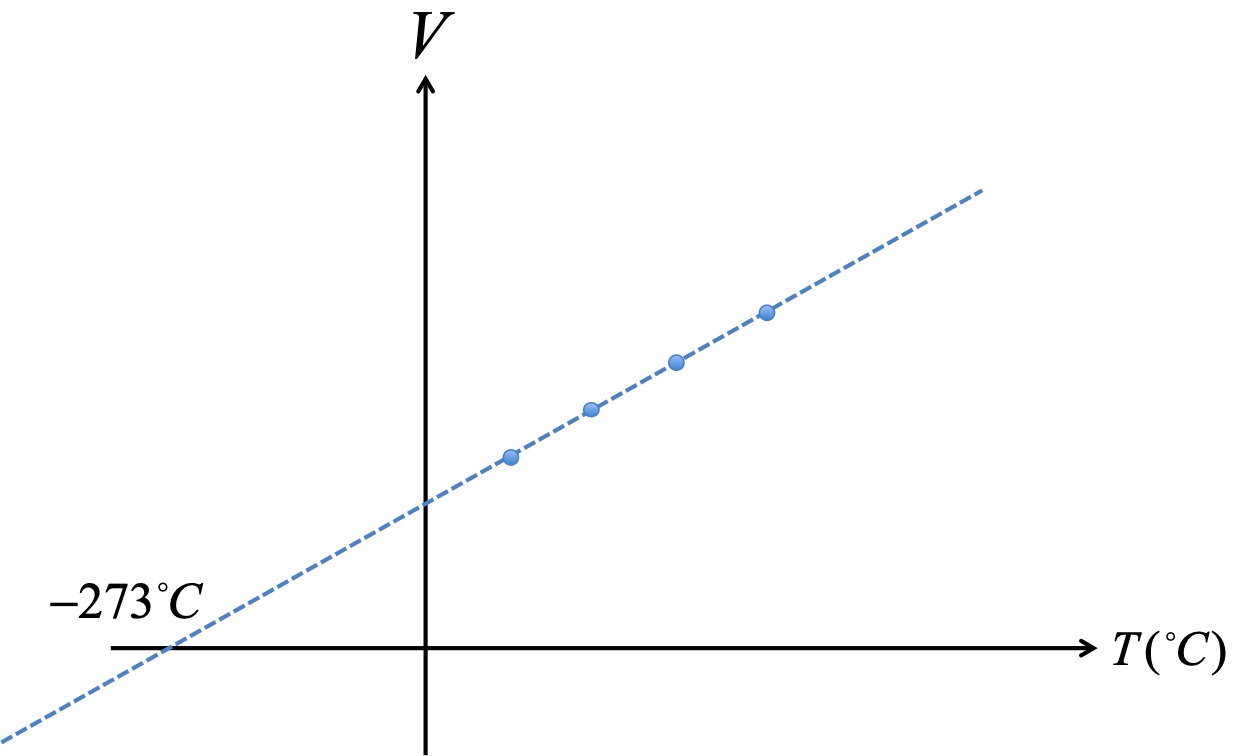
The Pressure And Absolute Temperature . The practical formula for the combined gas law gives “before and after” conditions of a gas: The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The unit of the kelvin scale is the kelvin (k), named. P1v1 / t1 = p2v2 / t2. Graph of pressure versus temperature: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero.
from dept.swccd.edu
The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero. Graph of pressure versus temperature: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: P1v1 / t1 = p2v2 / t2. The unit of the kelvin scale is the kelvin (k), named. The practical formula for the combined gas law gives “before and after” conditions of a gas: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas.
Lecture 16 Temperature and Theory
The Pressure And Absolute Temperature The unit of the kelvin scale is the kelvin (k), named. The unit of the kelvin scale is the kelvin (k), named. Graph of pressure versus temperature for various gases kept at a constant volume. P1v1 / t1 = p2v2 / t2. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Note that all of the graphs extrapolate to zero. Graph of pressure versus temperature: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The practical formula for the combined gas law gives “before and after” conditions of a gas:
From www.chegg.com
Solved Part II. Pressure and Absolute Temperature The Pressure And Absolute Temperature P1v1 / t1 = p2v2 / t2. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The practical formula for the combined gas law gives “before and after” conditions of a gas: The unit of the kelvin scale is the kelvin (k), named. The physical law that relates the pressure. The Pressure And Absolute Temperature.
From brainly.com
Which graph shows the pressuretemperature relationship for a gas at a The Pressure And Absolute Temperature P1v1 / t1 = p2v2 / t2. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. The volume of. The Pressure And Absolute Temperature.
From schematichonorable.z21.web.core.windows.net
Tv Diagram Constant Pressure The Pressure And Absolute Temperature The practical formula for the combined gas law gives “before and after” conditions of a gas: P1v1 / t1 = p2v2 / t2. Graph of pressure versus temperature: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. This relationship. The Pressure And Absolute Temperature.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. Graph of pressure versus temperature: P1v1 / t1 = p2v2 / t2. The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of. The Pressure And Absolute Temperature.
From www.coursehero.com
[Solved] The pressure and temperature at the beginning of compression The Pressure And Absolute Temperature The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. Graph of pressure versus temperature for various gases kept at a constant volume. Graph of pressure versus temperature: The volume of a given gas sample is directly proportional to its. The Pressure And Absolute Temperature.
From slideplayer.com
I. Physical Properties (p ) ppt download The Pressure And Absolute Temperature Graph of pressure versus temperature: The unit of the kelvin scale is the kelvin (k), named. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature for various gases. The Pressure And Absolute Temperature.
From saylordotorg.github.io
Relationships among Pressure, Temperature, Volume, and Amount The Pressure And Absolute Temperature The unit of the kelvin scale is the kelvin (k), named. The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature for various gases kept at a constant volume. The physical law that relates the pressure and volume of a gas to the number of gas molecules or number. The Pressure And Absolute Temperature.
From saylordotorg.github.io
Relationships among Pressure, Temperature, Volume, and Amount The Pressure And Absolute Temperature The unit of the kelvin scale is the kelvin (k), named. The practical formula for the combined gas law gives “before and after” conditions of a gas: The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The physical law that relates the pressure and volume of a gas to the. The Pressure And Absolute Temperature.
From byjus.com
How does the volume of gas vary with its absolute temperature, at The Pressure And Absolute Temperature The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature for various gases kept at a constant volume. P1v1 / t1 = p2v2 / t2. Note. The Pressure And Absolute Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel The Pressure And Absolute Temperature This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The practical formula for the combined gas law gives “before and after” conditions of a gas: P1v1 / t1 = p2v2 / t2. The unit of the kelvin scale is the kelvin (k), named. Note that all of the graphs. The Pressure And Absolute Temperature.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. P1v1 / t1 = p2v2 / t2. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature for various gases kept. The Pressure And Absolute Temperature.
From dept.swccd.edu
Lecture 16 Temperature and Theory The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). P1v1 / t1 = p2v2 / t2. The physical law that relates the pressure and volume of a gas to the. The Pressure And Absolute Temperature.
From ct-stem.northwestern.edu
CTSTEM The Pressure And Absolute Temperature Graph of pressure versus temperature: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. The physical. The Pressure And Absolute Temperature.
From www.numerade.com
SOLVED Which of the following graphs shows the correct relationship The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. P1v1 / t1 = p2v2 / t2. Graph of pressure versus temperature for various gases kept at a constant volume. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The physical law that relates the pressure and volume of a gas. The Pressure And Absolute Temperature.
From blog.beamex.com
Temperature units and temperature unit conversion The Pressure And Absolute Temperature This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. The volume of a given gas sample. The Pressure And Absolute Temperature.
From plot.ly
Graph showing Temperature Vs Absolute Pressure scatter chart made by The Pressure And Absolute Temperature The practical formula for the combined gas law gives “before and after” conditions of a gas: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer,. The Pressure And Absolute Temperature.
From hxeidmcjj.blob.core.windows.net
If The Pressure And Absolute Temperature at Ellen Lopes blog The Pressure And Absolute Temperature The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero. Graph of pressure versus temperature: The physical law that relates the pressure and volume of a gas to the number of gas. The Pressure And Absolute Temperature.
From www.physics.brocku.ca
PPLATO FLAP PHYS 7.2 Temperature, pressure and the ideal gas laws The Pressure And Absolute Temperature Graph of pressure versus temperature: P1v1 / t1 = p2v2 / t2. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Note that all of the graphs extrapolate to zero. The practical formula for the combined gas law gives “before and after” conditions of a gas: The volume of. The Pressure And Absolute Temperature.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Graph of pressure versus temperature for various gases kept at a constant volume. P1v1 / t1 = p2v2 / t2. The. The Pressure And Absolute Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel The Pressure And Absolute Temperature The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature: The unit of the kelvin scale is the kelvin. The Pressure And Absolute Temperature.
From ambientweather.com
Ambient Weather Support The Pressure And Absolute Temperature The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature for various gases kept at a constant volume. P1v1 / t1 = p2v2 / t2. This relationship between pressure and. The Pressure And Absolute Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel The Pressure And Absolute Temperature The practical formula for the combined gas law gives “before and after” conditions of a gas: The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. The unit of the kelvin scale is the kelvin (k), named. The volume of. The Pressure And Absolute Temperature.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 The Pressure And Absolute Temperature Graph of pressure versus temperature: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature for various gases kept at a constant volume. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). The physical law. The Pressure And Absolute Temperature.
From www.chegg.com
Solved Part II. Pressure and Absolute Temperature The Pressure And Absolute Temperature This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Note that all of the graphs extrapolate to zero. Graph of pressure versus temperature for various gases kept at a constant volume. The unit of the kelvin scale is the kelvin (k), named. The physical law that relates the pressure. The Pressure And Absolute Temperature.
From www.coyoteents.com
TEMPERATURE AND TIRE PRESSURE Coyote Enterprises The Pressure And Absolute Temperature P1v1 / t1 = p2v2 / t2. The unit of the kelvin scale is the kelvin (k), named. Graph of pressure versus temperature: The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Graph of pressure versus temperature for various gases kept at a constant volume. The physical law that relates. The Pressure And Absolute Temperature.
From brainly.in
the pressure and absolute temperature of certain mass of gas doubled The Pressure And Absolute Temperature P1v1 / t1 = p2v2 / t2. Graph of pressure versus temperature: Note that all of the graphs extrapolate to zero. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). Graph of pressure versus temperature for various gases kept at a constant volume. The unit of the kelvin scale is. The Pressure And Absolute Temperature.
From www.youtube.com
Thermodynamics Absolute Pressure and Absolute Temperature, STP & NTP The Pressure And Absolute Temperature Note that all of the graphs extrapolate to zero. P1v1 / t1 = p2v2 / t2. Graph of pressure versus temperature for various gases kept at a constant volume. The practical formula for the combined gas law gives “before and after” conditions of a gas: Graph of pressure versus temperature: The physical law that relates the pressure and volume of. The Pressure And Absolute Temperature.
From www.chegg.com
Part II Pressure and Absolute Temperature We've The Pressure And Absolute Temperature Graph of pressure versus temperature for various gases kept at a constant volume. The practical formula for the combined gas law gives “before and after” conditions of a gas: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature: P1v1 / t1 = p2v2 /. The Pressure And Absolute Temperature.
From www.template.net
Temperature Pressure Chart in Illustrator, PDF Download The Pressure And Absolute Temperature Graph of pressure versus temperature for various gases kept at a constant volume. The practical formula for the combined gas law gives “before and after” conditions of a gas: This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Graph of pressure versus temperature: The unit of the kelvin scale. The Pressure And Absolute Temperature.
From www.researchgate.net
Relationships between the gas temperature, absolute humidity and water The Pressure And Absolute Temperature The physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas. The unit of the kelvin scale is the kelvin (k), named. Graph of pressure versus temperature: P1v1 / t1 = p2v2 / t2. Note that all of the graphs extrapolate. The Pressure And Absolute Temperature.
From chart-studio.plotly.com
Boyle's Law PressureVolume Relationship in Gases scatter chart made The Pressure And Absolute Temperature Graph of pressure versus temperature for various gases kept at a constant volume. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The unit of the kelvin scale is the kelvin (k), named. The physical law that relates the pressure and volume of a gas to the number of. The Pressure And Absolute Temperature.
From www.chegg.com
TABLE C.1 Physical Properties of Air at Standard The Pressure And Absolute Temperature The unit of the kelvin scale is the kelvin (k), named. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). P1v1 / t1 = p2v2 / t2. The practical formula for the combined gas law gives “before and after” conditions of a gas: The physical law that relates the pressure. The Pressure And Absolute Temperature.
From socratic.org
Which graph shows the relationship between the temperature and volume The Pressure And Absolute Temperature P1v1 / t1 = p2v2 / t2. Note that all of the graphs extrapolate to zero. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The unit of the kelvin scale is the kelvin (k), named. Graph of pressure versus temperature: The physical law that relates the pressure and. The Pressure And Absolute Temperature.
From www.savemyexams.com
Gases & Absolute Temperature Cambridge O Level Physics Revision Notes The Pressure And Absolute Temperature The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). P1v1 / t1 = p2v2 / t2. Note that all of the graphs extrapolate to zero. The unit of the kelvin scale is the kelvin (k), named. This relationship between pressure and volume is known as boyle’s law, after its discoverer,. The Pressure And Absolute Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download The Pressure And Absolute Temperature The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles’s law). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Note that all of the graphs extrapolate to zero. The physical law that relates the pressure and volume of a gas. The Pressure And Absolute Temperature.