Aluminum Oxidation Number . Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Find the oxidation numbers of the elements in a chemical formula using this online tool. Oxidation is the loss of electrons and reduction is the gain of electrons. It has a +3 oxidation state and forms many alloys and. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Enter the formula, a net ionic charge, or an element. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (.
from studylib.net
In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. It has a +3 oxidation state and forms many alloys and. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Oxidation is the loss of electrons and reduction is the gain of electrons. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Enter the formula, a net ionic charge, or an element. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions.
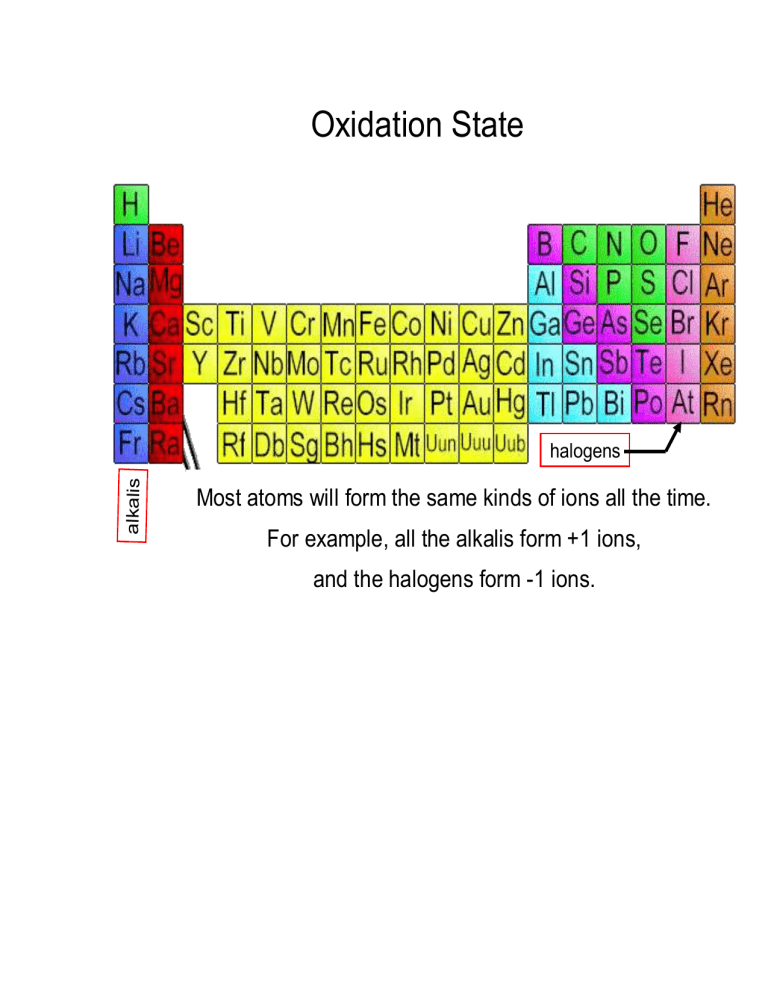
Oxidation Numbers
Aluminum Oxidation Number Oxidation is the loss of electrons and reduction is the gain of electrons. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. It has a +3 oxidation state and forms many alloys and. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Find the oxidation numbers of the elements in a chemical formula using this online tool. Enter the formula, a net ionic charge, or an element. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Oxidation is the loss of electrons and reduction is the gain of electrons.
From www.museoinclusivo.com
Understanding Aluminum Oxidation Number Overview, Applications and Aluminum Oxidation Number Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and. Aluminum Oxidation Number.
From www.slideserve.com
PPT WarmUp PowerPoint Presentation, free download ID3731178 Aluminum Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. It has a +3 oxidation state and forms many alloys and. Enter the formula, a net ionic charge, or an element. Learn how to identify. Aluminum Oxidation Number.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. The oxidation state of. Aluminum Oxidation Number.
From utedzz.blogspot.com
Labeled Periodic Table With Oxidation Numbers Periodic Table Timeline Aluminum Oxidation Number Enter the formula, a net ionic charge, or an element. Oxidation is the loss of electrons and reduction is the gain of electrons. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn. Aluminum Oxidation Number.
From www.museoinclusivo.com
Understanding Aluminum Oxidation Number Overview, Applications and Aluminum Oxidation Number In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Find the oxidation numbers of the elements in a chemical formula using this online tool. Enter the formula, a net ionic charge, or an element. Oxidation is the loss of electrons and reduction is the gain of electrons. It has. Aluminum Oxidation Number.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Aluminum Oxidation Number Oxidation is the loss of electrons and reduction is the gain of electrons. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. It has. Aluminum Oxidation Number.
From www.priyamstudycentre.com
Oxidation Number Periodic table elements Definition, Rules Aluminum Oxidation Number Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. It has a +3 oxidation state and forms many alloys and. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Oxidation is the loss of electrons and reduction is. Aluminum Oxidation Number.
From www.chegg.com
Solved 8. Assign oxidation numbers to all of the elements in Aluminum Oxidation Number Oxidation is the loss of electrons and reduction is the gain of electrons. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. The oxidation state of an atom is equal to the total. Aluminum Oxidation Number.
From educationspares.z4.web.core.windows.net
What Is Aluminum's Oxidation Number Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. It has a +3 oxidation state and forms many alloys and. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Oxidation is the loss of electrons and reduction is the gain of electrons. In order to. Aluminum Oxidation Number.
From www.museoinclusivo.com
What is the Oxidation Number of Aluminum? A Comprehensive Guide Aluminum Oxidation Number Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Find the. Aluminum Oxidation Number.
From sciencenotes.org
Downloadable Periodic Table Oxidation States Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn how to determine. Aluminum Oxidation Number.
From www.youtube.com
How To Calculate Oxidation Numbers Basic Introduction YouTube Aluminum Oxidation Number It has a +3 oxidation state and forms many alloys and. Enter the formula, a net ionic charge, or an element. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal. Aluminum Oxidation Number.
From shiken.ai
Oxidation Number Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive. Aluminum Oxidation Number.
From www.bartleby.com
Answered The oxidation number of aluminum in… bartleby Aluminum Oxidation Number Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Oxidation is the loss of electrons and reduction is the gain of electrons. It has a +3 oxidation state and forms many alloys and. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn about the properties,. Aluminum Oxidation Number.
From www.slideserve.com
PPT Elements and Periodic Table PowerPoint Presentation, free Aluminum Oxidation Number Oxidation is the loss of electrons and reduction is the gain of electrons. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Learn the definition and rules of oxidation numbers, and how. Aluminum Oxidation Number.
From www.museoinclusivo.com
Understanding Aluminum Oxidation Number Overview, Applications and Aluminum Oxidation Number Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Aluminum Oxidation Number.
From www.museoinclusivo.com
What is the Oxidation Number of Aluminum? A Comprehensive Guide Aluminum Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. In order to. Aluminum Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for Al2O3 (Aluminum oxide) YouTube Aluminum Oxidation Number Enter the formula, a net ionic charge, or an element. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. It has a +3 oxidation state and forms many alloys and. Oxidation is the. Aluminum Oxidation Number.
From sciencenotes.org
How to Assign Oxidation Numbers Aluminum Oxidation Number Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Enter the formula, a net ionic charge, or an element. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. In order to understand all types of redox reactions, you must have a way to determine the. Aluminum Oxidation Number.
From www.museoinclusivo.com
What is the Oxidation Number of Aluminum? A Comprehensive Guide Aluminum Oxidation Number Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. The oxidation state. Aluminum Oxidation Number.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. It has a +3 oxidation state and forms many alloys and. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Learn how to identify and assign oxidation states to atoms in chemical compounds,. Aluminum Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Aluminum Oxidation Number Oxidation is the loss of electrons and reduction is the gain of electrons. It has a +3 oxidation state and forms many alloys and. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. In order to understand all types of redox reactions, you must have a way to determine. Aluminum Oxidation Number.
From www.numerade.com
SOLVED The oxidation number of aluminum in Al3+ is Aluminum Oxidation Number Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. It has a +3 oxidation state and forms many alloys and. Oxidation is the loss of electrons and reduction is the gain of electrons. In order to understand all types of redox reactions, you must have a way to determine the oxidation number. Aluminum Oxidation Number.
From barkmanoil.com
What Is The Oxidation Number Of Nitrogen In Aluminum Nitride, Aln? The Aluminum Oxidation Number Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. It has a +3 oxidation state and forms many alloys and. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. In order to understand all types of redox reactions, you must have a. Aluminum Oxidation Number.
From studylib.net
Oxidation Numbers Aluminum Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Find the oxidation numbers of the elements in a chemical formula using this online tool. Oxidation is the loss of electrons and reduction is the gain of electrons. It has a +3 oxidation state and. Aluminum Oxidation Number.
From www.museoinclusivo.com
Understanding Aluminum Oxidation Number Overview, Applications and Aluminum Oxidation Number Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Oxidation is the loss of electrons and reduction is the gain of electrons. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. The oxidation state of an atom is. Aluminum Oxidation Number.
From www.museoinclusivo.com
Understanding Aluminum Oxidation Number Overview, Applications and Aluminum Oxidation Number Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Enter the formula,. Aluminum Oxidation Number.
From aluminumgenjin.blogspot.com
Aluminum Aluminum Oxidation Number Aluminum Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation. Oxidation is the loss of electrons and reduction is the gain of electrons. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Enter the. Aluminum Oxidation Number.
From www.youtube.com
008 Aluminum + Oxygen = Aluminum Oxide YouTube Aluminum Oxidation Number In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. It has a +3 oxidation state and forms many alloys and. The oxidation state of an atom is equal to the total number of. Aluminum Oxidation Number.
From aluminumgenjin.blogspot.com
Aluminum Oxidation Number Of Aluminum Aluminum Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. Oxidation is the loss of electrons and reduction is the gain of electrons. The oxidation state of an atom is equal to the total number. Aluminum Oxidation Number.
From www.scribd.com
Common Oxidation Numbers Chart Aluminum Oxidation Number Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Learn how to determine the electron configuration and oxidation states of different elements based on their position in the periodic. Enter the formula,. Aluminum Oxidation Number.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Aluminum Oxidation Number Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Enter the formula, a net ionic charge, or an element. It has a +3 oxidation state and forms many alloys and. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. In order to understand all types. Aluminum Oxidation Number.
From study.com
Oxidation Number Definition, Rules & Examples Video & Lesson Aluminum Oxidation Number It has a +3 oxidation state and forms many alloys and. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Learn the definition and rules of oxidation numbers, and how to use them to follow chemical reactions and name compounds. Learn about the properties, history, compounds and reactions of aluminum, the most. Aluminum Oxidation Number.
From www.chemeurope.com
Periodic table of oxidations states Aluminum Oxidation Number Enter the formula, a net ionic charge, or an element. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Learn how to identify and assign oxidation states to atoms in chemical compounds, especially in redox reactions. Learn how to determine the electron configuration and oxidation states of different elements based on their position. Aluminum Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Aluminum Oxidation Number In order to understand all types of redox reactions, you must have a way to determine the oxidation number (. It has a +3 oxidation state and forms many alloys and. Oxidation is the loss of electrons and reduction is the gain of electrons. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn. Aluminum Oxidation Number.