Chlorine Isotopes Protons Neutrons Electrons . All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Only two chlorine isotopes exist in. The arrangements of electrons above the last (closed shell) noble gas. This is approximately the sum of the number of protons and neutrons. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It has an atomic weight of 35.450 and a mass.
from ar.inspiredpencil.com
The arrangements of electrons above the last (closed shell) noble gas. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. This is approximately the sum of the number of protons and neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. There are two stable isotopes, 35 cl. It has an atomic weight of 35.450 and a mass. Only two chlorine isotopes exist in. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17.
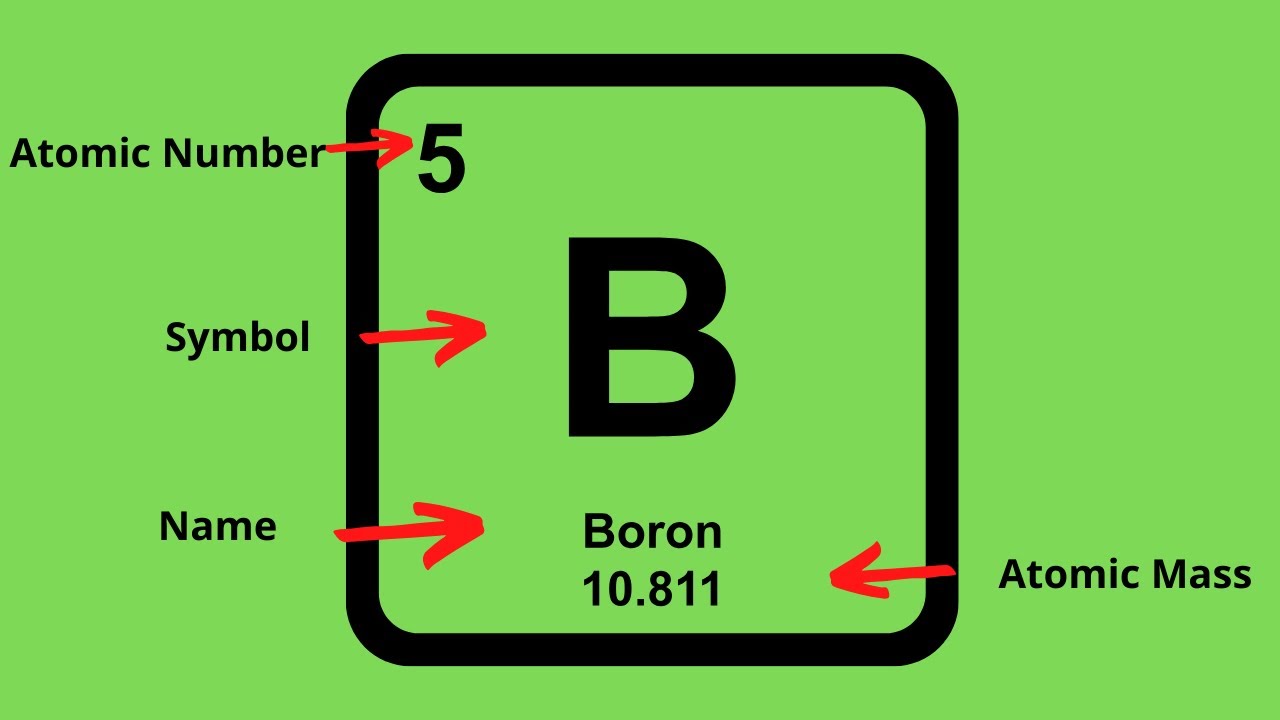
Periodic Table Of Elements With Protons Neutrons And Electrons
Chlorine Isotopes Protons Neutrons Electrons It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. It has an atomic weight of 35.450 and a mass. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. The arrangements of electrons above the last (closed shell) noble gas. This is approximately the sum of the number of protons and neutrons. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Only two chlorine isotopes exist in. There are two stable isotopes, 35 cl.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total. Chlorine Isotopes Protons Neutrons Electrons.
From manuallistcantabank.z21.web.core.windows.net
Hydrogen Atomic Diagram Chlorine Isotopes Protons Neutrons Electrons It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. This is approximately the sum of the number of protons. Chlorine Isotopes Protons Neutrons Electrons.
From exokgjdvg.blob.core.windows.net
Protons Neutrons And Electrons In Chlorine 35 at David Spears blog Chlorine Isotopes Protons Neutrons Electrons It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. This is approximately the sum of the. Chlorine Isotopes Protons Neutrons Electrons.
From lessonfullparanoics.z13.web.core.windows.net
Calculating Isotopes Worksheets Chlorine Isotopes Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Only two chlorine isotopes exist in. This is approximately the sum of the number of protons and neutrons. It has an atomic weight of 35.450 and a mass. There are two stable isotopes, 35 cl. Chlorine is a chemical element with atomic number 17 which means there. Chlorine Isotopes Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. The arrangements of electrons above the last (closed shell) noble gas. There are two stable isotopes, 35 cl. Only two chlorine isotopes exist in. It has an atomic weight of 35.450 and a mass. Chlorine. Chlorine Isotopes Protons Neutrons Electrons.
From diagrambomenslikpo.z13.web.core.windows.net
Bohr Rutherford Diagram Of Chlorine Chlorine Isotopes Protons Neutrons Electrons 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. There are two stable isotopes, 35 cl. This is approximately the sum of. Chlorine Isotopes Protons Neutrons Electrons.
From awesomehome.co
Using The Periodic Table To Determine Protons Neutrons And Electrons Chlorine Isotopes Protons Neutrons Electrons It has an atomic weight of 35.450 and a mass. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons,. Chlorine Isotopes Protons Neutrons Electrons.
From www.numerade.com
SOLVED How many protons; neutrons and electrons are there in a neutral Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. It has an atomic weight of 35.450 and a mass. The arrangements of electrons above the last (closed shell) noble gas. This is approximately the sum of the number of protons and neutrons. Chlorine is the 17th element in the periodic. Chlorine Isotopes Protons Neutrons Electrons.
From msoid.ibuypower.com
Introduction And Terminology Worksheet Answer Key Best Printable Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. There are two stable isotopes, 35 cl. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. The arrangements of electrons above the last (closed shell) noble gas. Only two chlorine isotopes exist in. All atoms of chlorine. Chlorine Isotopes Protons Neutrons Electrons.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Isotopes Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. All atoms of chlorine (cl) have 17 protons,. Chlorine Isotopes Protons Neutrons Electrons.
From www.alamy.com
Carbon isotopes. Atomic Structure from Carbon12 to Carbon14. Atomic Chlorine Isotopes Protons Neutrons Electrons The arrangements of electrons above the last (closed shell) noble gas. It has an atomic weight of 35.450 and a mass. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. This is approximately the sum of the number of protons and neutrons. Chlorine is the 17th. Chlorine Isotopes Protons Neutrons Electrons.
From lessonlistcarolyn.z21.web.core.windows.net
Protons Neutrons Electrons Worksheet Chlorine Isotopes Protons Neutrons Electrons There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Only two chlorine isotopes exist in. This is approximately the sum of the number of protons and neutrons. Chlorine is a chemical element with atomic number 17 which means there. Chlorine Isotopes Protons Neutrons Electrons.
From www.slideserve.com
PPT Atomic Symbols and Isotopes PowerPoint Presentation, free Chlorine Isotopes Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. The arrangements of electrons above the last (closed shell) noble gas. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers,. Chlorine Isotopes Protons Neutrons Electrons.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Chlorine Isotopes Protons Neutrons Electrons Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17. Chlorine Isotopes Protons Neutrons Electrons.
From periodictable.me
Na+Symbols+Find+the+1++number+of+protons+number+of+neutrons Dynamic Chlorine Isotopes Protons Neutrons Electrons There are two stable isotopes, 35 cl. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Only two. Chlorine Isotopes Protons Neutrons Electrons.
From nl.wikihow.com
Het aantal neutronen, protonen en elektronen bepalen wikiHow Chlorine Isotopes Protons Neutrons Electrons There are two stable isotopes, 35 cl. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. It has an atomic weight of 35.450 and a mass. This is approximately the sum of the number of protons. Chlorine Isotopes Protons Neutrons Electrons.
From exokabuml.blob.core.windows.net
Chlorine Electrons Neutrons And Protons at Wanda Palm blog Chlorine Isotopes Protons Neutrons Electrons 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. This is approximately the sum of the number of protons and neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine. Chlorine Isotopes Protons Neutrons Electrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. The arrangements of electrons above the last (closed shell) noble gas. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two. Chlorine Isotopes Protons Neutrons Electrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Chlorine Isotopes Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. There are two stable isotopes, 35 cl. It has. Chlorine Isotopes Protons Neutrons Electrons.
From awesomehome.co
Using The Periodic Table To Determine Protons Neutrons And Electrons Chlorine Isotopes Protons Neutrons Electrons Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. It has an atomic weight of 35.450 and a mass. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl. Chlorine Isotopes Protons Neutrons Electrons.
From allen.in
CBSE Notes for Class 9 Science Chapter 4 Structure of the Atom Chlorine Isotopes Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. This is approximately the sum of the number of protons and neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. There are two stable isotopes, 35 cl. It has an atomic weight of 35.450 and a mass.. Chlorine Isotopes Protons Neutrons Electrons.
From www.theknowledgelibrary.in
What is an Isotope? The Knowledge Library Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. There are two stable isotopes, 35 cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. It has an atomic weight of 35.450 and a mass. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine. Chlorine Isotopes Protons Neutrons Electrons.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. It has an atomic weight of 35.450 and a mass. There are two stable isotopes, 35 cl. The arrangements of electrons above the last (closed shell) noble gas. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. All atoms of chlorine (cl) have. Chlorine Isotopes Protons Neutrons Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Isotopes Protons Neutrons Electrons 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. This is approximately the sum of the number of protons and neutrons. There are two stable isotopes,. Chlorine Isotopes Protons Neutrons Electrons.
From londonkruwmerritt.blogspot.com
How Many Protons Electrons and Neutrons Does Chlorine Have Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of. This is approximately the sum of the number of protons and neutrons. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It has. Chlorine Isotopes Protons Neutrons Electrons.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. 53 rows chlorine. Chlorine Isotopes Protons Neutrons Electrons.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Protons Neutrons Electrons This is approximately the sum of the number of protons and neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. There are two stable isotopes, 35 cl. It has an atomic weight of 35.450 and a mass. The arrangements of electrons above the last (closed shell) noble gas. All atoms of chlorine (cl) have 17. Chlorine Isotopes Protons Neutrons Electrons.
From www.energy.gov
Understanding the Outsized Effect of Hydrogen Isotopes Department of Chlorine Isotopes Protons Neutrons Electrons Only two chlorine isotopes exist in. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons, but there are. Chlorine Isotopes Protons Neutrons Electrons.
From www.chegg.com
C. Subatomic Particles\table[[Element,Atomic Chlorine Isotopes Protons Neutrons Electrons 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23. Chlorine Isotopes Protons Neutrons Electrons.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine Isotopes Protons Neutrons Electrons The arrangements of electrons above the last (closed shell) noble gas. It has an atomic weight of 35.450 and a mass. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in. Chlorine Isotopes Protons Neutrons Electrons.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Isotopes Protons Neutrons Electrons Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. There are two stable isotopes, 35 cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.total number of.. Chlorine Isotopes Protons Neutrons Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Isotopes Protons Neutrons Electrons There are two stable isotopes, 35 cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Only two chlorine isotopes exist in. It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine is the 17th element in. Chlorine Isotopes Protons Neutrons Electrons.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Protons Neutrons Electrons 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. There are two stable isotopes, 35 cl. This is approximately the sum of the number of protons and neutrons. Only two chlorine isotopes exist. Chlorine Isotopes Protons Neutrons Electrons.