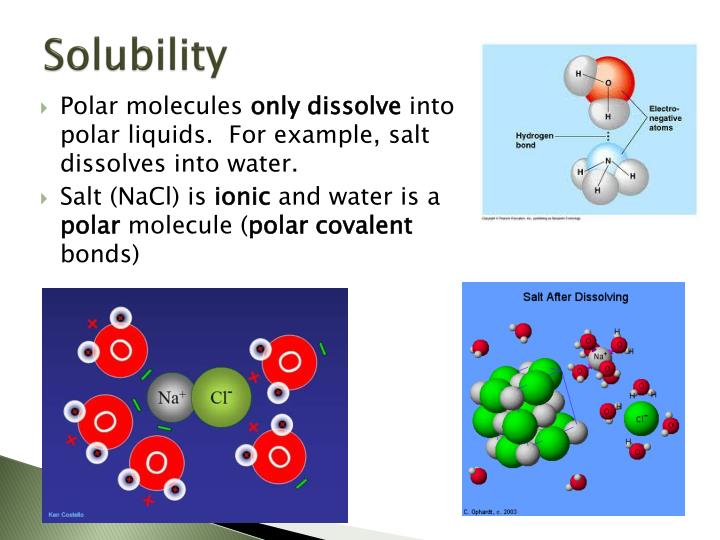
Which Substances To Dissolve In Water Ionic Or Covalent . Covalent bonds form between nonmetals. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Nonelectrolytes are substances that do not produce ions when. Water typically dissolves most ionic compounds and polar molecules. Substances that dissolve in water to yield ions are called electrolytes. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from.
from www.slideserve.com
Nonpolar molecules such as those found in grease or oil do not dissolve in water. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Substances that dissolve in water to yield ions are called electrolytes. Covalent bonds form between nonmetals. Nonelectrolytes are substances that do not produce ions when. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves many ionic compounds and polar molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't.
PPT Bonding PowerPoint Presentation ID3050946
Which Substances To Dissolve In Water Ionic Or Covalent Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Substances that dissolve in water to yield ions are called electrolytes. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Water typically dissolves many ionic compounds and polar molecules. Covalent bonds form between nonmetals.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Water typically dissolves many ionic compounds and polar molecules. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonpolar. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Which Substances To Dissolve In Water Ionic Or Covalent Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Water typically dissolves most ionic compounds and polar molecules. Substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ions being surrounded by water molecules helps to stabilize aqueous. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.science-revision.co.uk
Covalent bonding Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Covalent bonds form between nonmetals. Nonpolar molecules, such as those found in grease or oil, do not. Which Substances To Dissolve In Water Ionic Or Covalent.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves most ionic compounds and polar molecules. Nonelectrolytes are substances that do not produce ions when. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Substances that dissolve. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Which Substances To Dissolve In Water Ionic Or Covalent Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Water typically dissolves many ionic compounds and polar molecules. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water.. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves many ionic compounds and polar molecules.. Which Substances To Dissolve In Water Ionic Or Covalent.
From slideplayer.com
Chapter 4 Formation of Compounds ppt download Which Substances To Dissolve In Water Ionic Or Covalent If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water. Which Substances To Dissolve In Water Ionic Or Covalent.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves many ionic compounds and polar molecules. Covalent bonds form between nonmetals. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Ions being surrounded by water molecules helps to stabilize aqueous. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Which Substances To Dissolve In Water Ionic Or Covalent The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Nonelectrolytes are substances that do not produce ions when. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves most ionic compounds and polar molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Water typically dissolves many ionic compounds and polar molecules. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. If the attraction between the ions. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves many ionic compounds and polar molecules. If the attraction between the ions and the water molecules. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Formation of a precipitate PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Water typically dissolves many. Which Substances To Dissolve In Water Ionic Or Covalent.
From examples.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Nonpolar molecules,. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves most ionic compounds and polar molecules. Water typically dissolves many ionic compounds and polar molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Ions being surrounded. Which Substances To Dissolve In Water Ionic Or Covalent.
From blogs.glowscotland.org.uk
Review Comparing Ionic/ Covalent Compounds S3 Chemistry Consolidation Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Covalent bonds form between nonmetals. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonelectrolytes are substances that do not produce ions when. Substances that dissolve in water to yield. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Homogeneous and Heterogeneous Mixtures PowerPoint Presentation Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. Water typically dissolves most ionic compounds and polar molecules. Water typically dissolves many ionic compounds and polar molecules. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. If the attraction between the ions and the water molecules is great enough to break the. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.shutterstock.com
4 Ionic Vs. Covalent Compounds Images, Stock Photos & Vectors Which Substances To Dissolve In Water Ionic Or Covalent Substances that dissolve in water to yield ions are called electrolytes. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Properties of Ionic, Covalent, and Metallic Compounds PowerPoint Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond.. Which Substances To Dissolve In Water Ionic Or Covalent.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Which Substances To Dissolve In Water Ionic Or Covalent Nonelectrolytes are substances that do not produce ions when. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Water typically dissolves many ionic compounds and polar molecules. Covalent bonds form between nonmetals. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Substances. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves many ionic compounds and polar molecules. Substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ions being surrounded by water molecules helps to stabilize aqueous. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Which Substances To Dissolve In Water Ionic Or Covalent Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. The bond. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Formation of a precipitate PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules such as those found in grease or oil do not dissolve in water. Water typically dissolves many ionic compounds and polar molecules. Covalent bonds form between nonmetals. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Ions being surrounded by water molecules helps. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.numerade.com
SOLVED Many ionic and covalent compounds dissolve well in water Which Substances To Dissolve In Water Ionic Or Covalent Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves most ionic compounds and polar molecules. Nonelectrolytes are substances that do not produce ions when. Water typically dissolves many ionic compounds and polar molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Covalent bonds form between nonmetals. The bond. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules such as those found in grease or oil do not dissolve in water. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Covalent bonds form between nonmetals. Substances. Which Substances To Dissolve In Water Ionic Or Covalent.
From courses.lumenlearning.com
The Structure and Properties of Water Introduction to Chemistry Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Water typically dissolves most ionic compounds and polar molecules. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Water typically dissolves many ionic compounds and polar. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Covalent bonds form between nonmetals. Substances that dissolve in water to yield ions are called electrolytes. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. If the attraction between the ions and the water molecules. Which Substances To Dissolve In Water Ionic Or Covalent.
From slideplayer.com
Molarity and Dilutions Photo by Chris ppt download Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves many ionic compounds and polar molecules. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. The bond between hydrogen and oxygen atoms in. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free Which Substances To Dissolve In Water Ionic Or Covalent Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Covalent bonds form between nonmetals.. Which Substances To Dissolve In Water Ionic Or Covalent.
From slideplayer.com
Types of covalent bonds ppt download Which Substances To Dissolve In Water Ionic Or Covalent Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Covalent bonds form between nonmetals. Nonelectrolytes are substances that do not produce ions when. Water typically dissolves most ionic compounds and polar molecules. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the. Which Substances To Dissolve In Water Ionic Or Covalent.
From study.com
Compound Solubility in Water Overview & Examples Lesson Which Substances To Dissolve In Water Ionic Or Covalent Nonelectrolytes are substances that do not produce ions when. Covalent bonds form between nonmetals. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Nonpolar molecules, such as those found in grease or oil, do not. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Which Substances To Dissolve In Water Ionic Or Covalent Water typically dissolves most ionic compounds and polar molecules. Water typically dissolves many ionic compounds and polar molecules. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together,. Which Substances To Dissolve In Water Ionic Or Covalent.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Ionic compounds conduct electricity when melted. Which Substances To Dissolve In Water Ionic Or Covalent.
From brightideas.houstontx.gov
Create A Visual Model Of An Ionic Substance (salt) Dissolving In Water Which Substances To Dissolve In Water Ionic Or Covalent Covalent bonds form between nonmetals. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from.. Which Substances To Dissolve In Water Ionic Or Covalent.
From sciencenotes.org
Covalent Compounds Examples and Properties Which Substances To Dissolve In Water Ionic Or Covalent Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from. Nonelectrolytes are substances that do not produce ions when. Water typically dissolves most ionic compounds and polar molecules. Water typically dissolves many ionic compounds and polar molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically. Which Substances To Dissolve In Water Ionic Or Covalent.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Substances To Dissolve In Water Ionic Or Covalent Substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. The bond between hydrogen and oxygen atoms in water (h 2 o) is a good example of a polar covalent bond. Covalent bonds form between nonmetals. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Which Substances To Dissolve In Water Ionic Or Covalent.