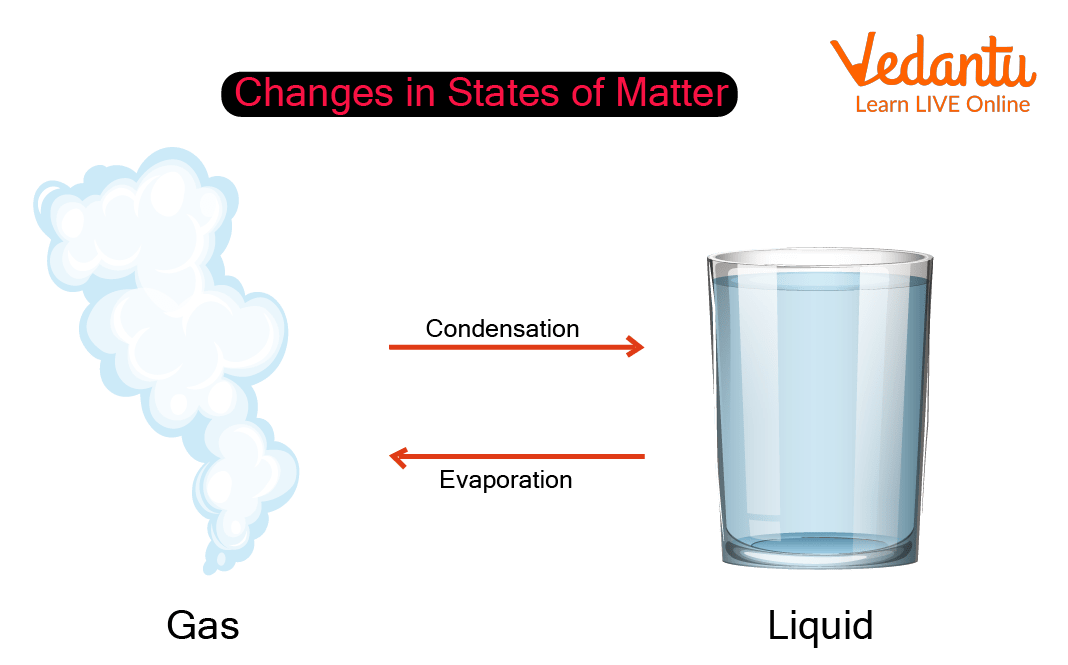
Example Heat Of Condensation . The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. This release of latent heat. When latent heat is released it is converted into. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a.
from www.vedantu.com
The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. This release of latent heat. When latent heat is released it is converted into. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid.
Evaporation and Condensation Definition, Applications and Difference
Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. When latent heat is released it is converted into. This release of latent heat. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo Example Heat Of Condensation We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. The molar. Example Heat Of Condensation.
From www.slideserve.com
PPT Ch. 17 Thermochemistry PowerPoint Presentation, free download Example Heat Of Condensation The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. The molar heat of condensation of a substance is the. Example Heat Of Condensation.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. This release of latent heat. When latent heat is released it is converted into. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that. Example Heat Of Condensation.
From www.animalia-life.club
Example Of Condensation Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. Condensation is the reverse process, where heat. Example Heat Of Condensation.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. During the condensation process, water molecules lose. Example Heat Of Condensation.
From ar.inspiredpencil.com
Example Of Deposition Gas To Solid Example Heat Of Condensation We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. This release of latent heat. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released. Example Heat Of Condensation.
From www.slideserve.com
PPT Phases, Phase Changes, Chemical and Physical Changes PowerPoint Example Heat Of Condensation We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. This release of latent heat. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released. Example Heat Of Condensation.
From www.slideserve.com
PPT Changes in State of Matter PowerPoint Presentation, free download Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. This release of latent heat. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. During the condensation process, water molecules lose the 600 cal/gm of latent heat that. Example Heat Of Condensation.
From jeopardylabs.com
REVIEW of PHYSICAL STATES (solid, liquid, gas) Jeopardy Template Example Heat Of Condensation When latent heat is released it is converted into. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),.. Example Heat Of Condensation.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Example Heat Of Condensation When latent heat is released it is converted into. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted. Example Heat Of Condensation.
From www.ck12.org
Heat of vaporization Example 1 ( Video ) Chemistry CK12 Foundation Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. This release of latent heat. During. Example Heat Of Condensation.
From brainly.ph
example of condensation Brainly.ph Example Heat Of Condensation The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The heat \(q\) required to change the. Example Heat Of Condensation.
From www.slideserve.com
PPT Cool Condensation PowerPoint Presentation, free download ID2835918 Example Heat Of Condensation The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. We have seen that vaporization requires heat. Example Heat Of Condensation.
From www.studyiq.com
Evaporation and Condensation, Definition, Process, Types, Difference Example Heat Of Condensation The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole. Example Heat Of Condensation.
From www.slideserve.com
PPT Essential Question How do particles behave in the four states of Example Heat Of Condensation Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. When latent heat is released it is converted into. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of vaporization (δ h vap) of. Example Heat Of Condensation.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Example Heat Of Condensation This release of latent heat. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The heat. Example Heat Of Condensation.
From www.slideserve.com
PPT Water States of Matter PowerPoint Presentation, free download Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. This release of latent heat. When latent heat is released it is converted into. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance. Example Heat Of Condensation.
From www.vedantu.com
Evaporation and Condensation Definition, Applications and Difference Example Heat Of Condensation The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. When latent heat is released it is converted into. Condensation. Example Heat Of Condensation.
From www.snexplores.org
Explainer What are the different states of matter? Example Heat Of Condensation During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. This release of latent heat. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. Condensation is the reverse process, where heat. Example Heat Of Condensation.
From definitionvde.blogspot.com
What Is The Definition Of Latent Heat DEFINITIONVD Example Heat Of Condensation This release of latent heat. Condensation is the reverse process, where heat in transferred away from a substance to its surroundings. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. During the condensation process, water molecules lose the 600 cal/gm of latent heat. Example Heat Of Condensation.
From lavelle.chem.ucla.edu
Difference CHEMISTRY COMMUNITY Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. This release of latent heat. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The. Example Heat Of Condensation.
From vectormine.com
Condensation explanation as water droplets formation on glass outline Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. The molar heat of vaporization (δ h vap) of a substance is the. Example Heat Of Condensation.
From www.thoughtco.com
How Condensation and Evaporation Shape Our Weather Example Heat Of Condensation The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released. Example Heat Of Condensation.
From ar.inspiredpencil.com
Condensation Science Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. This release of latent heat. We have seen that. Example Heat Of Condensation.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. When latent heat is released it. Example Heat Of Condensation.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The molar heat of condensation of. Example Heat Of Condensation.
From www.geeksforgeeks.org
What is Vaporization? Example Heat Of Condensation During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. This release of latent heat. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. When latent heat is released it is. Example Heat Of Condensation.
From www.mindomo.com
Physical Geography of Canada Mind Map Example Heat Of Condensation The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. We have seen that vaporization requires heat. Example Heat Of Condensation.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid. The heat \(q\) required to change. Example Heat Of Condensation.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Example Heat Of Condensation The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. When latent heat is released it is converted into.. Example Heat Of Condensation.
From animalia-life.club
Example Of Condensation Example Heat Of Condensation When latent heat is released it is converted into. This release of latent heat. The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. The molar heat of vaporization (δ h vap) of a substance is the heat absorbed by one mole of that substance. Example Heat Of Condensation.
From ar.inspiredpencil.com
Condensation Science Example Heat Of Condensation This release of latent heat. When latent heat is released it is converted into. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. The. Example Heat Of Condensation.
From slideplayer.com
Heat in Changes of State ppt download Example Heat Of Condensation When latent heat is released it is converted into. This release of latent heat. The heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\) (evaporating or condensing),. During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process.. Example Heat Of Condensation.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help Example Heat Of Condensation We have seen that vaporization requires heat transfer to a liquid from the surroundings, so that energy is released by the surroundings. The heat \(q\) required to change the phase of a sample of mass \(m\) is given by \[ q = ml_f (melting/freezing),\] \[q = ml_v. This release of latent heat. The molar heat of condensation of a substance. Example Heat Of Condensation.
From www.youtube.com
Heat Transfer L28 p2 Condensation RealWorld Examples YouTube Example Heat Of Condensation During the condensation process, water molecules lose the 600 cal/gm of latent heat that were added during the evaporation process. The molar heat of condensation \(\left( \delta h_\text{cond} \right)\) is the heat released by one mole of a substance as it is converted from a. The heat q required to change the phase of a sample of mass m is. Example Heat Of Condensation.