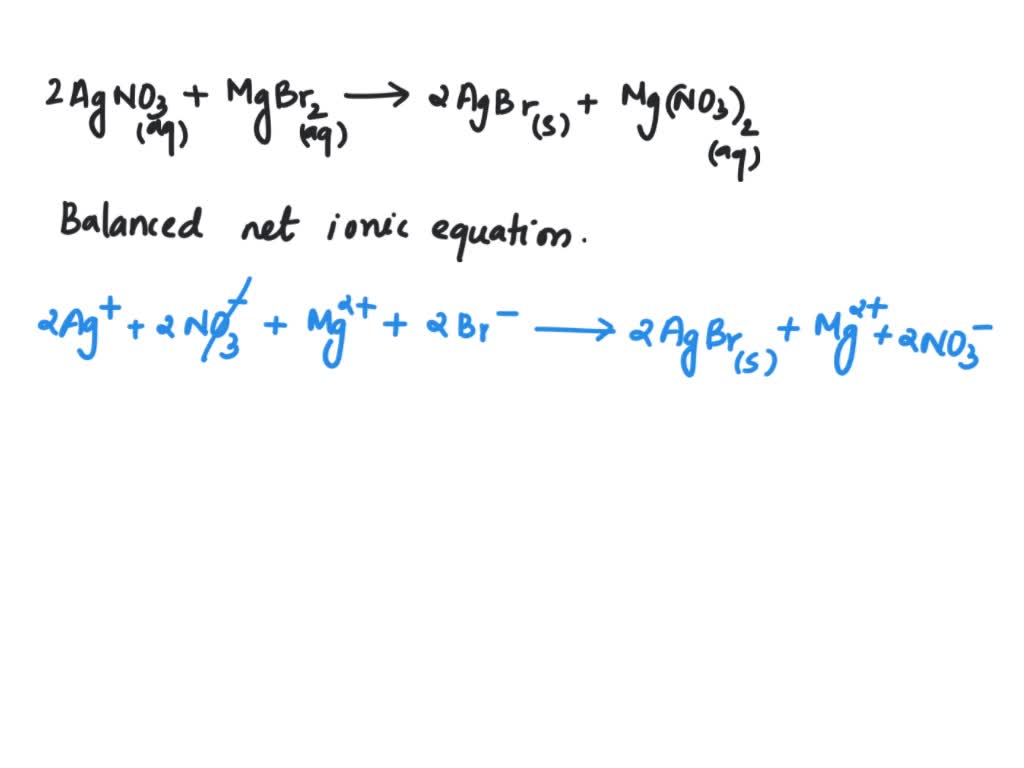Magnesium Chloride And Silver Nitrate . Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. The balanced equation will be calculated along. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Next, we predict the possible products of the reaction using the principle of double displacement.
from www.numerade.com
As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Next, we predict the possible products of the reaction using the principle of double displacement. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous.
SOLVED The following molecular equation represents the reaction that
Magnesium Chloride And Silver Nitrate As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Next, we predict the possible products of the reaction using the principle of double displacement. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Magnesium Chloride And Silver Nitrate Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Next, we predict the possible products of the reaction using the principle of double displacement. The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. As an example, silver nitrate. Magnesium Chloride And Silver Nitrate.
From www.youtube.com
Double displacement KCl+AgNO3 Potassium chloride + Silver nitrate Magnesium Chloride And Silver Nitrate Next, we predict the possible products of the reaction using the principle of double displacement. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Enter an equation of an ionic. Magnesium Chloride And Silver Nitrate.
From www.coursehero.com
[Solved] 6,881 g of a sample containing magnesium chloride and sodium Magnesium Chloride And Silver Nitrate Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. As an example, silver nitrate and sodium chloride react to form sodium. Magnesium Chloride And Silver Nitrate.
From www.sciencecompany.com
Reagent Grade Silver Nitrate AgNO3, 10g for sale. Buy from The Science Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. Next,. Magnesium Chloride And Silver Nitrate.
From www.carolina.com
Magnesium Nitrate, Hexahydrate, Reagent Grade, 500 g Carolina Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Aqueous solutions. Magnesium Chloride And Silver Nitrate.
From www.numerade.com
Silver Nitrate reacts with Magnesium Chloride to produce Silver Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Next,. Magnesium Chloride And Silver Nitrate.
From www.stargrace-magnesite.com
China Magnesium Nitrate Formula Suppliers, Producer, Manufacturers Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. The balanced equation will be calculated along. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products. Magnesium Chloride And Silver Nitrate.
From www.sxleixinchem.com
China Magnesium Nitrate Crystal Powder Manufacturers, Suppliers Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Aqueous. Magnesium Chloride And Silver Nitrate.
From www.slideserve.com
PPT Write down the formulae for Silver chloride Sodium hydroxide Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the. Magnesium Chloride And Silver Nitrate.
From www.youtube.com
The Reaction Between Silver Nitrate and Sodium Chloride YouTube Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. Next, we predict the possible products of the reaction using the principle of double displacement. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride. Magnesium Chloride And Silver Nitrate.
From www.slideserve.com
PPT Fig. 3.11 PowerPoint Presentation, free download ID981677 Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous.. Magnesium Chloride And Silver Nitrate.
From askfilo.com
I Reactions and Equations Silver nitrate + Sodium chloride Silver chlor.. Magnesium Chloride And Silver Nitrate When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. The balanced equation will be calculated along. Next, we predict the possible products of the reaction using the principle of double displacement. Enter an equation of an ionic chemical equation and press the balance button. As an example, silver. Magnesium Chloride And Silver Nitrate.
From www.numerade.com
SOLVED The following molecular equation represents the reaction that Magnesium Chloride And Silver Nitrate Next, we predict the possible products of the reaction using the principle of double displacement. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Enter an equation of an ionic chemical equation and press the balance button. As an example, silver nitrate and sodium chloride react to form. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved When solutions of silver nitrate and magnesium Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride.. Magnesium Chloride And Silver Nitrate.
From pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Next, we predict the possible products of the reaction using the principle of double displacement. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved When solutions of silver nitrate and magnesium Magnesium Chloride And Silver Nitrate Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Enter an equation of an ionic chemical equation and press the balance. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved What is the net ionic equation for the reaction that Magnesium Chloride And Silver Nitrate Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved (15p) Silver Nitrate reacts with Magnesium Chloride Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Next,. Magnesium Chloride And Silver Nitrate.
From www.nanochemazone.com
Magnesium Nitrate Powder Price 1 Nanochemazone Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Next, we predict the possible products of the reaction using the principle of double displacement. The balanced equation will be calculated along. As an example, silver. Magnesium Chloride And Silver Nitrate.
From www.shalom-education.com
Testing for Sulfate Ions GCSE Chemistry Revision Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Next, we predict the possible products of. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved 41. Silver nitrate, AgNO3(aq), reacts with iron(III) Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Aqueous solution of magnesium chloride and silver nitrate are mixed to form. Magnesium Chloride And Silver Nitrate.
From alchetron.com
Magnesium nitrate Alchetron, The Free Social Encyclopedia Magnesium Chloride And Silver Nitrate As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. The balanced equation will be calculated along. Next, we predict the possible products of the reaction using the principle of double displacement. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate.. Magnesium Chloride And Silver Nitrate.
From www.youtube.com
WHEN SILVER NITRATE REACTS WITH MAGNESIUM RIBBON YouTube Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. Next, we predict the possible products of the reaction using the principle of double displacement. As an example, silver nitrate and. Magnesium Chloride And Silver Nitrate.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Magnesium Chloride And Silver Nitrate Next, we predict the possible products of the reaction using the principle of double displacement. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Aqueous solutions of magnesium chloride. Magnesium Chloride And Silver Nitrate.
From www.numerade.com
SOLVED MgCl2 (aq) + 2 AgNO3 (aq) > 2 AgCl (s) + Mg(NO3)2 (aq) How Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Enter an equation of an ionic chemical equation and press the balance button. Next, we predict the possible products of the reaction using the principle of double displacement. As an example, silver nitrate and sodium chloride react to form sodium nitrate. Magnesium Chloride And Silver Nitrate.
From www.youtube.com
How to Balance Mg + AgNO3 = Mg(NO3)2 + Ag (Magnesium + Silver nitrate Magnesium Chloride And Silver Nitrate When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. The balanced equation will be calculated along. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. Next, we predict the possible products of the reaction using the principle of. Magnesium Chloride And Silver Nitrate.
From slideplayer.com
Net Ionic Equations. ppt download Magnesium Chloride And Silver Nitrate Next, we predict the possible products of the reaction using the principle of double displacement. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. The balanced equation will. Magnesium Chloride And Silver Nitrate.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Chloride And Silver Nitrate Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous.. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved Magnesium metal reacts with aqueous silver nitrate Magnesium Chloride And Silver Nitrate Next, we predict the possible products of the reaction using the principle of double displacement. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and. Magnesium Chloride And Silver Nitrate.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium. Magnesium Chloride And Silver Nitrate.
From brainly.in
200ml of 0.05M magnesium chloride is mixed with 75ml of 0.1M silver Magnesium Chloride And Silver Nitrate As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Next, we predict the possible products of the reaction using the principle of double displacement. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Enter an equation of an ionic. Magnesium Chloride And Silver Nitrate.
From www.magnesiumchloridechina.com
Magnesium Chloride Powder Magnesium Chloride And Silver Nitrate As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. The balanced equation will be calculated along. Next, we. Magnesium Chloride And Silver Nitrate.
From 33772c03311619ec.en.made-in-china.com
Hot Sale and High Quality Magnesium Nitrate, Magnesium Nitrate Magnesium Chloride And Silver Nitrate The balanced equation will be calculated along. Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. When aqueous solutions of magnesium chloride (mgcl2) and silver nitrate (agno3) are mixed, the products are solid silver chloride and aqueous. Enter an equation of an ionic chemical equation and press the balance button.. Magnesium Chloride And Silver Nitrate.
From www.chegg.com
Solved Elver nitrate reacts with magnesium chloride in water Magnesium Chloride And Silver Nitrate Enter an equation of an ionic chemical equation and press the balance button. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. The balanced equation will be calculated along. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. Aqueous. Magnesium Chloride And Silver Nitrate.
From www.slideserve.com
PPT Honors chemistry ch . 14 Ions in Aqueous Solutions and Magnesium Chloride And Silver Nitrate Aqueous solution of magnesium chloride and silver nitrate are mixed to form solid silver chloride and aqueous magnesium nitrate. As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. Aqueous solutions of magnesium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous magnesium nitrate. The balanced. Magnesium Chloride And Silver Nitrate.