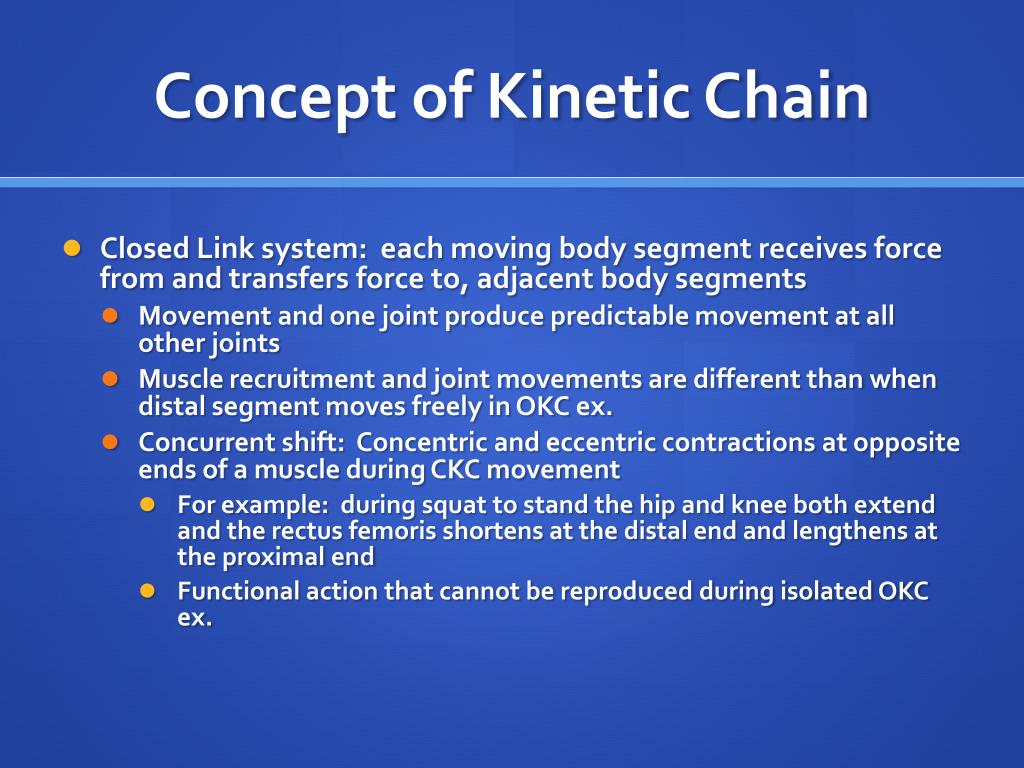
What Is Kinetic Chain Length . Ν = rate of chain growth = rate of chain growth. Kinetics can also be used to establish a theoretical polymer chain length. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetic chain length there will be some obvious errors (e.g. What about chains that were initiated, but did not terminate just before. Which implies instantaneous initiation of all chains uppon addition of catalyst. Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. In principle, it's just the ratio of the chain propagation rate to the chain initiation The kinetic chain length 〈x(t)〉 is the chain length or average. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. This quantity is called kinetic chain length, represented by vee bar.
from www.slideserve.com
The kinetic chain length 〈x(t)〉 is the chain length or average. In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetics can also be used to establish a theoretical polymer chain length. This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length there will be some obvious errors (e.g. Which implies instantaneous initiation of all chains uppon addition of catalyst. Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = # of monomers added per effective free radical.
PPT OpenVersus Chain Exercise in Rehabilitation
What Is Kinetic Chain Length Which implies instantaneous initiation of all chains uppon addition of catalyst. In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetic chain length there will be some obvious errors (e.g. This quantity is called kinetic chain length, represented by vee bar. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = rate of chain growth = rate of chain growth. What about chains that were initiated, but did not terminate just before. Which implies instantaneous initiation of all chains uppon addition of catalyst. The kinetic chain length 〈x(t)〉 is the chain length or average. Kinetics can also be used to establish a theoretical polymer chain length. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = # of monomers added per effective free radical.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID382916 What Is Kinetic Chain Length Kinetic chain length there will be some obvious errors (e.g. Which implies instantaneous initiation of all chains uppon addition of catalyst. Kinetics can also be used to establish a theoretical polymer chain length. Ν = # of monomers added per effective free radical. The kinetic chain length 〈x(t)〉 is the chain length or average. The expression for kinetic chain length. What Is Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System What Is Kinetic Chain Length The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. This quantity is called kinetic chain length, represented by vee bar. Which implies instantaneous initiation of all chains uppon addition of catalyst. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic chain. What Is Kinetic Chain Length.
From bodyinbalancept.com
The Concept of the Chain Body in Balance PT What Is Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length 〈x(t)〉 is the chain length or average. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic chain length there. What Is Kinetic Chain Length.
From www.youtube.com
Introduction to Polymers Lecture 6.6 Free radical polymerization What Is Kinetic Chain Length Ν = # of monomers added per effective free radical. The kinetic chain length 〈x(t)〉 is the chain length or average. What about chains that were initiated, but did not terminate just before. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = rate. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Ionic, RingOpening, & Controlled Polymerization PowerPoint What Is Kinetic Chain Length Ν = # of monomers added per effective free radical. Which implies instantaneous initiation of all chains uppon addition of catalyst. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. The kinetic chain length 〈x(t)〉 is the chain length or average. Ν = rate of chain growth = rate of chain growth. In principle, it's just the. What Is Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System What Is Kinetic Chain Length This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Kinetic chain length there will be some obvious errors (e.g. In principle, it's just the ratio of the chain propagation rate to the chain initiation Ν = rate. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Ionic, RingOpening, & Controlled Polymerization PowerPoint What Is Kinetic Chain Length Ν = # of monomers added per effective free radical. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation What about chains that were initiated, but did not terminate just before. The average. What Is Kinetic Chain Length.
From strengthandsoul.co.uk
What is Chain Release (KCR), can it help you? And why do we What Is Kinetic Chain Length What about chains that were initiated, but did not terminate just before. Which implies instantaneous initiation of all chains uppon addition of catalyst. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetics can also be used to establish a theoretical polymer chain length. Kinetic chain length there will be some obvious errors (e.g. This quantity is. What Is Kinetic Chain Length.
From mobilephysiotherapyclinic.in
Open Chain Exercise Characteristics, Benefits, Examples What Is Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = rate of chain growth = rate of chain growth. Kinetics can also be used to establish a theoretical polymer chain length. In principle, it's just the ratio of the chain propagation rate to the. What Is Kinetic Chain Length.
From blog.naver.com
까먹고 안 했네 닫힌 사슬운동(closed kinematic chain) 네이버 블로그 What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. What about chains that were initiated, but did not terminate just before. This quantity is called kinetic chain length, represented by vee bar. The kinetic chain length 〈x(t)〉 is the chain length or average. Which implies instantaneous initiation of all chains uppon addition of catalyst. Ν = #. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Ionic polymerization PowerPoint Presentation, free download ID What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. In principle, it's just the ratio of the chain propagation rate to the chain initiation Ν = # of monomers added per effective free radical. Ν = rate of chain growth = rate of chain growth. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2.. What Is Kinetic Chain Length.
From www.slideshare.net
Polymer Course What Is Kinetic Chain Length In principle, it's just the ratio of the chain propagation rate to the chain initiation The kinetic chain length 〈x(t)〉 is the chain length or average. What about chains that were initiated, but did not terminate just before. Ν = rate of chain growth = rate of chain growth. Which implies instantaneous initiation of all chains uppon addition of catalyst.. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Chap 9. Chaingrowth Polymerization PowerPoint Presentation, free What Is Kinetic Chain Length Kinetic chain length there will be some obvious errors (e.g. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Which implies instantaneous initiation of all chains uppon addition of catalyst. What about chains. What Is Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System What Is Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation Which implies instantaneous initiation of all chains uppon addition of catalyst. Kinetic chain length there will be some obvious errors (e.g. Ν = rate. What Is Kinetic Chain Length.
From www.doubtnut.com
Half length of the chain of mass m and length / is overhanging. What i What Is Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = rate of chain growth = rate of chain growth. Kinetic chain length there will be some obvious errors (e.g. Kinetic chain length refers to the average number of repeating units or monomers that are. What Is Kinetic Chain Length.
From bodybuilding-wizard.com
Closed and Open Chain Exercises • Bodybuilding Wizard What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. What about chains that were initiated, but did not terminate just before. Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic. What Is Kinetic Chain Length.
From www.slideshare.net
Chains • Open Chain What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. What about chains that were initiated, but did not terminate just before. The kinetic chain length 〈x(t)〉 is the chain length or average. Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to. What Is Kinetic Chain Length.
From www.sportsrec.com
What Is Chain Exercise? SportsRec What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Which implies instantaneous initiation of all chains uppon addition of catalyst. This quantity is called. What Is Kinetic Chain Length.
From massages4u.co.uk
MASSAGES 4U Chain Release brings the body back into balance What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. Which implies instantaneous initiation of all chains uppon addition of catalyst. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers. What Is Kinetic Chain Length.
From www.pinterest.com
PPT Chain Exercises Open vs . Closed Chain PowerPoint What Is Kinetic Chain Length In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length 〈x(t)〉 is the chain length or average. Ν = rate of chain growth = rate of chain growth. Which implies. What Is Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System What Is Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Which implies instantaneous initiation of all chains uppon addition of catalyst. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetic chain. What Is Kinetic Chain Length.
From www.restoreandresetripon.com
Chain Release What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. Ν = rate of chain growth = rate of chain growth. What about chains that were initiated, but did not terminate just before. This quantity is called kinetic chain length, represented by vee bar. The kinetic chain length 〈x(t)〉 is the chain length or average. The average number. What Is Kinetic Chain Length.
From www.youtube.com
What is Chain Release (KCR)? YouTube What Is Kinetic Chain Length The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Which implies instantaneous initiation of all chains uppon addition of catalyst. In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν. What Is Kinetic Chain Length.
From slideplayer.com
Chapter 2 Radical/Chain Polymerization ppt download What Is Kinetic Chain Length Ν = # of monomers added per effective free radical. Ν = rate of chain growth = rate of chain growth. What about chains that were initiated, but did not terminate just before. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length 〈x(t)〉 is. What Is Kinetic Chain Length.
From posturegeek.com
Open Chain vs Closed Chain What's the Difference? What Is Kinetic Chain Length Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. What about chains that were initiated, but did not terminate just before. Kinetics can also be used to. What Is Kinetic Chain Length.
From slainte.usli.com
The Chain Explained Fundamentals of Proper Movement USLI What Is Kinetic Chain Length The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. In principle, it's just the ratio of the chain propagation rate to the chain initiation What about chains that were initiated, but did not terminate just before. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain.. What Is Kinetic Chain Length.
From www.youtube.com
Thermal Initiation (Contd.), Molecular Weight and Chain Length What Is Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. Which implies instantaneous initiation of all chains uppon addition of catalyst. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length 〈x(t)〉 is the chain length or average. What about chains that were initiated,. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Chap 9. Chaingrowth Polymerization PowerPoint Presentation, free What Is Kinetic Chain Length What about chains that were initiated, but did not terminate just before. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. Kinetics can also be used to establish a theoretical polymer chain length. Ν = rate of chain growth = rate of chain growth. The kinetic chain length 〈x(t)〉 is the chain length or average. The average. What Is Kinetic Chain Length.
From www.topvelocity.net
Mastering The High Velocity Pitchers Chain Increase Pitching What Is Kinetic Chain Length In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetic chain length there will be some obvious errors (e.g. This quantity is called kinetic chain length, represented by vee bar. Ν = rate of chain growth = rate of chain growth. Which implies instantaneous initiation of all chains uppon addition of catalyst. Kinetic chain. What Is Kinetic Chain Length.
From www.researchgate.net
Schematic illustration of the chain theory. Download What Is Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = # of monomers added per effective free radical. The kinetic chain length 〈x(t)〉 is the chain length or average. The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. The average number of monomers reacting. What Is Kinetic Chain Length.
From www.slideserve.com
PPT OpenVersus Chain Exercise in Rehabilitation What Is Kinetic Chain Length Kinetics can also be used to establish a theoretical polymer chain length. Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length 〈x(t)〉 is the chain length or average. This quantity is. What Is Kinetic Chain Length.
From www.youtube.com
Honors Symposium 2021 Open Chain Exercises vs. Closed What Is Kinetic Chain Length The kinetic chain length 〈x(t)〉 is the chain length or average. Kinetic chain length there will be some obvious errors (e.g. In principle, it's just the ratio of the chain propagation rate to the chain initiation Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = rate. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Chap 9. Chaingrowth Polymerization PowerPoint Presentation, free What Is Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. This quantity is called kinetic chain length, represented by vee bar. Which implies instantaneous initiation of all chains uppon addition of catalyst. The kinetic chain length 〈x(t)〉 is the chain length or average. Ν = rate of chain growth. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID254280 What Is Kinetic Chain Length The expression for kinetic chain length is 𝑣 = 𝑘 𝑝 2. What about chains that were initiated, but did not terminate just before. Which implies instantaneous initiation of all chains uppon addition of catalyst. Ν = rate of chain growth = rate of chain growth. The kinetic chain length 〈x(t)〉 is the chain length or average. In principle, it's. What Is Kinetic Chain Length.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID254280 What Is Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length 〈x(t)〉 is the chain length or average. Which implies instantaneous initiation of all chains uppon addition of catalyst. Ν = # of monomers added per effective free radical. What about chains that were initiated, but. What Is Kinetic Chain Length.